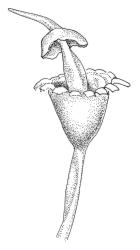
Elements in the following description are taken from Bartlett & Vitt (1986).
Plants medium-sized to robust, usually glossy and soft in texture, often yellow- or brown-green above and dark green to nearly black below, forming soft, densely interwoven mats or turves, aquatic, subaquatic, or on rocks in splash zones. Stems commonly much-branched by forking and innovation, wiry or less often soft, in cross-section with a central strand. Leaves erect-spreading or secund, sometimes falcate, usually little altered when dry, lanceolate or lanceolate-subulate, sometimes with a weakly defined oblong base, often appearing ± shouldered and this accentuated by the leaf concavity, the upper portion of the leaf ± filled by the costa, subtubulose below and clasping at base, plane at margins, entire or rarely denticulate above; upper laminal cells (in upper part of leaf base or ⅓ above insertion) elongate-rectangular to linear, rarely short-rectangular (in B. contecta), thick-walled, not or very weakly porose, smooth; lower laminal cells often longer; alar cells variably differentiated but usually coloured and inflated, sometimes remaining attached to stem. Costa single, narrow or broad below, mostly filling the subula, in cross-section usually with a differentiated median layer of guide cells.
Dioicous or autoicous. Perichaetial leaves with a defined base and ± abruptly shouldered, subulate. Perigonia on short lateral branches or terminal, with short, non-subulate bracts and mostly numerous antheridia intermixed with many filiform paraphyses. Setae variable in length, straight or curved; capsules erect, usually turbinate or urceolate when dry, ± hemispheric when moist, immersed to exserted, dark, usually thick-walled, often with a persistent columella; mouth transverse; exothecial cells thick-walled, sometimes thickened in corners; stomata few at base of capsule or sometimes appearing absent; annulus not differentiated; operculum mostly conic-rostrate or rarely bluntly mammillate (in B. martinii), often systylious; columella short or exserted. Peristome single, well-developed or absent, with 16, lanceolate, red to hyaline teeth, the outer surface composed of strongly thickened and usually smooth plates and with well-developed transverse walls (but sometimes appearing warty in N.Z. species due to preperistome development); preperistome sometimes present. Calyptra cucullate and smooth. Spores spherical, smooth or papillose, often green.
Blindia is a predominantly southern hemisphere genus of c. 20 species. Seven species are accepted from N.Z. A valuable survey of the genus in N.Z. was provided by Bartlett & Vitt (1986), while Andreas (2013) has recently published a revision for southern South America that treats several of our species. The N.Z. members of the genus most commonly occur on non-calcareous rocks, either irrigated or submerged, except for one species (B. martinii), which can occur on non-irrigated rock. There is a strong southern bias to the N.Z. distribution. Only three of the seven species have been found on the North I. where they are restricted to higher elevation sites.
The generic name Blindia was not used in early N.Z. literature, and this can lead to confusion concerning early collections which were assigned to other genera. The genus was erected in 1846 to accommodate the single widespread northern hemisphere B. acuta (Hedw.) Bruch. & Schimp. It was not until the early years of the 20th century that southern hemisphere species were assigned to the genus. Brotherus (1901–1909, p. 306) appears to have been the first to circumscribe Blindia in something like its modern sense. Dixon (1914) was the first to discuss the genus in a N.Z. context. The most recent revision of the genus was provided by Bartlett & Vitt (1986). Andreas (2013) provided a treatment for southern South America in which she described three new species. From her descriptions and illustrations, none of these three appear to be conspecific with N.Z. species.
Some N.Z. Blindia species show considerable variation, which makes the confident identification of a fraction of sterile collections difficult. The variability of B. robusta is particularly troublesome and some sterile material of this species can be very difficult to distinguish from either B. immersa or B. lewinskyae.
The isolated vegetative leaves of all N.Z. species of Blindia (with the exception of B. contecta and some populations of B. immersa) appear to have sharply differentiated leaf bases and subulae when viewed under the stereoscope. However in most N.Z. species, including B. magellanica, B. martinii, and B. robusta, the "shoulder" of the apparent leaf base is due primarily to the concavity of the leaves; if an isolated leaf is carefully flattened, the "shoulder" largely or entirely disappears. In most N.Z. species (including the three species just mentioned), perichaetial leaves are more distinctly shouldered than vegetative leaves, making the distinction between them critical. Therefore, when the laminal length or the lamina:subula ratio is used in keys and descriptions here, vegetative or perichaetial leaves are specified and the lamina is defined as that portion of the leaf in which laminal cells are visible in an unflattened leaf under the stereoscope. The subula is that portion of the leaf where laminal cells are not visible in an unflattened leaf under the stereoscope.
Bartlett & Vitt’s (1986) key to species (dichotomy 2) utilises lamina:subula length ratios to separate Blindia into two roughly equal-sized groups, with B. robusta keying out in both groups. This causes difficulty with sterile material, in part because the key does not specify whether the ratios were determined using perichaetial or vegetative leaves.
In the species where the vegetative leaves have no clearly defined leaf base, the term "upper laminal cells" is here used to describe cells taken ⅓ above the insertion, while in the species with a clearly differentiated leaf base, the upper laminal cells are described from the upper portion of the leaf base.
Although most N.Z. species have a clearly defined layer of median guide cells in a vegetative leaf costal cross-section (taken near the upper limit of the distinct lamina), the costae in B. acuta, the northern hemisphere generitype (vide J.A. Allen 69 from Washington State, CHR 412729), is homogeneous in cross-section.
The following key is modified from that of Bartlett & Vitt (1986).
| 1 | Lamina of vegetative leaves extending a third or more of the leaf length (under stereoscope); perichaetial leaves with a short subula that is 2 times or less the length of the leaf base | 2 |
| 1' | Lamina of vegetative leaves extending less than a third of the leaf length (under stereoscope); perichaetial leaves with an elongate subula that is usually 2–6 times the length of the leaf base | 5 |
| 2 | Capsules immersed in perichaetial leaves; vegetative leaves either denticulate at apices (in B. martinii) or cells of the upper lamina quadrate to short-rectangular (in B. contecta) | 3 |
| 2' | Capsules exserted beyond tips of perichaetial leaves; vegetative leaves entire throughout and cells of the upper lamina elongate | 4 |
| 3 | Vegetative leaves rigid; loosely erect to slightly secund; upper leaf margins entire; cells of upper lamina and of the abaxial surface of subula quadrate or short-rectangular, about 2:1; operculum long rostrate; plants usually submerged in streams and pools | B. contecta |
| 3' | Vegetative leaves falcate-secund to circinate-flexuose; upper leaf margins denticulate; cells of upper lamina and of the abaxial surface of subula elongate; operculum low-conic or mammillate, not rostrate; plants on irrigated rock, not submerged | B. martinii |
| 4 | Vegetative leaves mostly 3.0–5.0 mm, secund to ± falcate-secund but not circinate when moist; setae cygneous or strongly flexuose when moist; spores 18–24 µm | B. magellanica |
| 4' | Vegetative leaves mostly 6.0–9.0 mm, falcate to circinate, less often flexuose-secund or rarely ± straight when moist, setae stiffly erect or variably flexuose; spores 27–45 µm | B. robusta |
| 5 | Setae very short, <1.0 mm; capsules immersed; costa ill-defined and (150–)200–275 µm wide in leaf base, in cross-section lacking guide cells | B. immersa |
| 5' | Setae elongate; capsules immersed, emergent, or exserted; costa mostly well-defined and <100 µm wide in leaf base, in cross-section with a median layer of guide cells | 6 |
| 6 | Vegetative leaves 9.0–12.0 mm; capsules immersed to emergent; alar cells poorly developed; restricted to Nelson & Westland L.D. | B. lewinskyae |
| 6' | Vegetative leaves shorter, (5.0–)6.0–8.0(–9.0) mm; capsules ± exserted (usually clearly so); alar cells usually well-developed; widespread | 7 |
| 7 | Alar cells well-developed and usually visible in isolated vegetative leaves; costae mostly 50–60 µm wide and well-defined in lower leaf; vegetative leaves mostly distinctly falcate-secund (except when growing in still or slowly moving water); widely distributed on main islands | B. robusta |
| 7' | Alar cells not differentiated in vegetative leaves; costae usually 70–90 µm wide and ill-defined in lower leaf; vegetative leaves flexuose to loosely falcate-secund; known only from Auckland, Campbell, and Macquarie Is | B. seppeltii |
While confusion between Blindia and other subaquatic mosses is possible, members of the genus generally present a distinctive facies, even when sterile. When leaves are stripped from the stem, leaf base fragments often persist on the stem in a way that gives it a distinctive roughened appearance. In the species with differentiated alar cells, the pigmented, abruptly differentiated, and auriculate alar group is distinctive. The terminal position of sex organs and associated innovations also aid in distinguishing this genus from some superficially similar genera, including members of the Amblystegiaceae.
When sterile, more robust species of Blindia could be confused with aberrant material of Dicranoloma, particularly if the latter is growing submerged, as occasionally occurs. The stems of Blindia species are usually wiry and darkly coloured, with leaf bases adherent after leaves are removed, and have sparse (if any) rhizoids. The alar cells are mostly strongly pigmented and the leaves are mostly entire. The costae lack any spines or wings on the abaxial surface, and in cross-section are very simple in their anatomy (with a median band of guide cells) and mostly fill the subulae. The upper portion of the leaf thus lacks a distinct lamina. Species of Dicranoloma, by contrast, have softer and paler stems, usually lack adherent leaf bases, and are usually covered with dense pale-brown or whitish rhizoids. The alar cells there are often unpigmented and the leaf margins are mostly clearly toothed; the costae are often ornamented abaxially with spines or wings, and in cross-section have a more complex anatomy. The upper portion of the leaf in species of Dicranoloma nearly always has a distinct lamina.
The rare and high-elevation Holodontium strictum (Hook.f. & Wilson) Ochyra (=Chorisodontium burrowsii Allison) could easily be misinterpreted as a robust species of Blindia. However, H. strictum can be distinguished from Blindia spp. by the presence of papillae on the adaxial surface of its laminal cells and the lack of a central strand, among other features. The strongly lustrous, narrowly lanceolate, and falcate-secund leaves of the very rare Sematophyllum fiordensis Fife are suggestive of a Blindia, but its branching pattern and the absence of both costae and a central strand preclude confusion.
| Category | Number |
|---|---|
| Indigenous (Endemic) | 3 |
| Indigenous (Non-endemic) | 4 |
| Total | 7 |
Blindia maxwellii Vitt was described from Campbell I. According to Seppelt (2004, p. 152), B. maxwellii "bears a close similarity" to what he termed the "short seta form of D[itrichum] strictum from Macquarie Island". I have examined the holotype of B. maxwellii (NY 267838) and consider it to be a Ditrichum. It is, in my opinion, taxonomically the same as the the polster- forming Ditrichum from Macquarie I. in which, quoting Seppelt, "the seta reaches only around 1.0 cm in length and the capsule is barely exserted above the tips of the perichaetial leaves". The best name to apply to this Ditrichum, which occurs on Auckland, Campbell, and Macquarie Is, is unclear. Dale Vitt (pers. comm. 1 June 2015) has suggested that it may eventually prove to belong in the poorly understood genus Verrucidens.