- ≡ Blindia antarctica Müll.Hal., Syn. Musc. Frond. 1, 344 (1848)
- = Blindia chrysea Müll.Hal. in Beckett, Trans. & Proc. New Zealand Inst. 25: 290 (1893)
- = Weissia webbii R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 440 (1899)
- = Dicranum mackayi Broth. & Dixon in Dixon, J. Linn. Soc., Bot. 40: 437 (1912)
- ≡ Holodontium mackayi (Broth. & Dixon) Broth., Nat. Pflanzenfam., ed. 2 [Engler & Prantl] 10, 199 (1924)
- ≡ Dicranoweisia mackayi (Broth. & Dixon) Broth., Nat. Pflanzenfam., ed. 2 [Engler & Prantl] 10, 198 (1924)
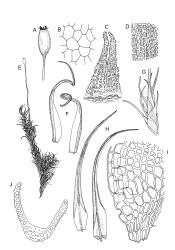
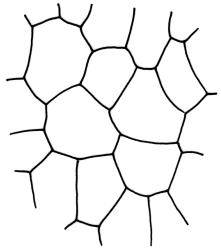
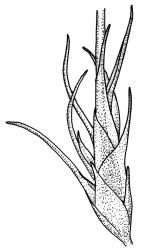
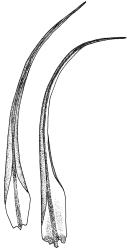
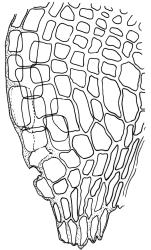
Plants medium-sized, forming dull brown-green cushions on rock. Stems c. 15–25(–40) mm, in cross-section with a distinct central strand and firm-walled cortical cells, with sparse, smooth, dark brown rhizoids restricted to the base. Leaves mostly loosely secund, sometimes ± falcate when moist, strongly cork-screwed when dry, narrowly lanceolate, entire, c. 3.5–4.0 mm, strongly concave to tubulose; mid to upper laminal cells short-rectangular or quadrate, sometimes oblate near margin, c. 4–6 µm wide and mostly 1–3:1, incrassate, unistratose, with longitudinal striations, which often extend across cells walls and appear as crenulations of the cell wall in cross-section (more conspicuous on abaxial surface); cells of lower leaf linear and thick-walled, weakly porose, lacking striations; alar cells ± inflated, unistratose, forming a strongly differentiated and weakly auriculate group extending to the costa, brown. Costa ± ill-defined below, occupying < ⅙ the leaf base, not filling the subula except at extreme apex, shortly excurrent, in cross-section (mid leaf) with median guide cells and both abaxial and adaxial stereid groups. Axillary hairs not seen.
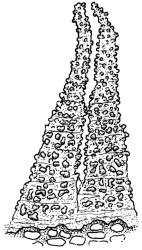
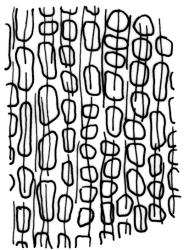
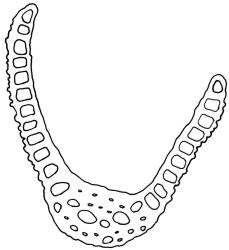
Paroicous. Perichaetial leaves c. 2.0–2.5 mm, the innermost with a tubulose and oblong base that sheathes the lower seta and a subula ⅓–½ the leaf length. Perigonia budlike at base of perichaetia, with filiform paraphyses, sometimes reduced to a single broadly ovate bract with axillary antheridia and paraphyses. Setae (4–)8–15(–22) mm, weakly flexuose, slender, weakly sinistrorse when dry, pale yellow-brown; capsules short-cylindric or obovoid, 1.0–1.8 mm, narrowed to the mouth, smooth (but becoming wrinkled with age); exothecial cells mostly oblong-hexagonal, not in columns, thin-walled; stomata absent or few, restricted to base, apparently superficial; annulus absent; operculum ± equalling the capsule, falling early. Peristome teeth orange throughout, fugacious, inserted c. 45–60 µm below rim, c. 200 × 40 µm (extending c. 150–160 µm beyond rim), lanceolate, very coarsely baculate throughout on both surfaces. Spores 15–24 µm, green, finely papillose.
Beckett 1893, plate 34 (as Blindia chrysea); Malcolm & Malcolm 2003, p. 22; Seppelt 2004, fig. 95.
Dicranoweisia antarctica could be confused with the generally larger Holomitrium perichaetiale, particularly when the latter species is growing on rock. As well as being smaller, D. antarctica lacks the subtle sheen on the abaxial surface of the costa seen in the Holomitrium. In D. antarctica the vegetative leaves are narrower, lack a distinct shoulder, and are strongly twisted in a cork-screw-like manner when dry, while in the Holomitrium the broader leaves are more distinctly shouldered and curved towards the stem when dry. Somewhat more difficult to observe are differences in sexuality (paroicous in the present species) and the length and shape (c. 2.0–2.5 mm and gradually tapered in the present species) of the perichaetial leaves; microscopically the cuticular striations of the laminal cells in the present species also separate it from H. perichaetiale.
Dicranoweisia antarctica differs from Kiaeria pumila in a number of features, including its undivided peristome teeth, which are coarsely and irregularly baculate (compared to the deeply split and longitudinally striate teeth of K. pumila), upper laminal cells that are shorter, thicker-walled, and striate, and the cork-screw twisting of its leaves when dry.
Sterile material of D. antarctica is also difficult to differentiate from the rare Kiaeria spenceri; the former nearly always grows on rock, while the latter appears to be exclusively corticolous. The gametophytes of the two are a similar yellow-green when fresh, but the leaves of the Dicranoweisia are longer (c. 4 mm) and obviously contorted when dry, while those of the Kiaeria are c. 2.5 mm, weakly secund, and not or only slightly contorted when dry. There are numerous sporophytic differences, including the nature of the peristome teeth, which distinguish the two species; these are detailed under K. spenceri.
Sporophytic characters readily distinguish D. antarctica from the N.Z. species of Amphidium. Dicranoweisia antarctica has exserted peristomate capsules in striking contrast to the immersed to weakly exserted and gymnostomous capsules of Amphidium. In the absence of sporophytes the distinction of these two genera is more difficult, at least in the field. The leaves of D. antarctica are more strongly cork-screwed than those of either species of Amphidium, and the alar cells form a well-differentiated group while those of Amphidium do not.
NI: Gisborne (Mt Hikurangi), Hawke’s Bay (Kaweka Range), Taranaki (Mt Taranaki), Wellington (Mt Ruapehu, Ruahine Range, Mt Holdsworth, Mt Hector, summit of Rimutaka Road, Turakirae Head); SI: Nelson, Marlborough, Canterbury, Westland, Otago, Southland; A; C; M.
Austral. Mainland Australia (Vic.*), Patagonia*, South Shetland Is.*, South Orkney Is.* Recorded from Tasmania by Sainsbury (1955a) and by Dalton et al. (1991).
Forming cushions from 10 to >100 mm diam. on non-calcareous rock (greywacke, gneiss, conglomerate, etc.) or on soil over rock, often in very exposed sites. Especially common in subalpine and alpine areas and often well-developed on streamside boulders. Rarely on exposed tree roots. The species is widespread in all the L.D. of the South I., but is less well-documented on the North I. The absence of records from Stewart I. may be a collection artefact. On the North I. recorded from low elevation (at Turakirae Head) and c. 600 m (summit of Rimutaka Road) to 1830 m (Mt Ruapehu). On South I. ranging from c. 140 m (near Dunedin) to 2550 m (Mt Cook, Canterbury L.D.). Occurring with a wide range of epilithic bryophyte species, including Andreaea acuminata, Brachythecium paradoxum, Breutelia spp., Ditrichum spp., Racomitrium crispulum s.l., Rhacocarpus purpurascens, Schistidium apocarpum, Frullania spp., Gackstroemia weindorferi, and Jamesoniella colorata.
Although Dicranoweisia antarctica varies greatly in stature, the characteristic cork-screw twisting of the dry vegetative leaves generally permits recognition in the field. The longitudinal striations of the leaf cells are highly diagnostic of the genus but sometimes can be obscure in surface view. The striations are sometimes clearer if leaves are stained with toluidine blue or a similar stain. The erect capsules with undivided peristome teeth are frequently produced.
The complicated nomenclatural history of this species was summarised by Bell (1976), who nevertheless did not typify the basionym, Blindia antarctica Müll.Hal. Müller (1848–1849, p. 344) described B. antarctica using two syntypes collected by J.D. Hooker from "Insula Campbelli et Eremitae ad Cap. Horn"; his description could be applied to either collection. The Hermite I. material (BM) is a homogeneous collection and corresponds well to modern concepts (Sainsbury 1955a; Bell 1976) of the species. One duplicate in BM-Hampe is labelled "Blindia antarctica mihi" in Müller’s hand, and is therefore selected here as the most appropriate lectotype. The Campbell I. syntype includes two dicranaceous species. A small fraction of sterile material is certainly conspecific with the Hermite I. material, but the bulk of the specimen lacks the characteristic cork-screw-twisted leaves and the cuticular striations characteristic of Dicranoweisia antarctica. None of the Campbell I. material in BM was annotated by Müller, and the bulk of this collection does not correspond to a modern concept of D. antarctica. The Campbell I. material thus cannot be accepted as the lectotype, despite its having been so annotated by R. Ochyra (in herb. BM).
Ochyra (1998, p. 122) applied the name Dicranoweisia brevipes (Müll.Hal.) Cardot to material from the South Shetland Is, but this is considered here to be D. antarctica. The South Shetland and South Orkney material available for study is sparsely fruiting or sterile. When capsules are present, the seta length is at the low end of the range of continuous variation observed for D. antarctica, but the material is otherwise representative of the species.
Too little is known of the Kerguelen Dicranoweisia antarctica var. robusta (Cardot) Seppelt for comment here. Dicranoweisia microcarpa (Hook.f. & Wilson) Paris is a poorly known Tasmanian species accepted by Dalton et al. (1991). This species is discussed by Sainsbury (1955b) and by Scott & Stone (1976, p. 158); it is briefly discussed and illustrated by Meagher & Fuhrer (2003), who recorded it from Victoria as well as Tasmania. It needs to be critically compared to the more widespread D. antarctica.