- ≡ Cheilanthes dicksonioides Endl., Prodr. Fl. Norfolk. 15 (1833)
- ≡ Hypolepis endlicheriana C.Presl, Tent. Pterid. 162 (1836) nom. illeg., nom. nov. pro Cheilanthes dicksonioides Endl. 1833
- = Cheilanthes pellucida Colenso, Tasmanian J. Nat. Sci. 2: 173 (1845)
- ≡ Hypolepis tenuifolia var. pellucida (Colenso) Hook., Sp. Fil. 2, 60, t. 90a (1858)
Rhizomes long-creeping, 3–8 mm diameter, with stipes arising 35–200 mm apart, or rarely 10 mm apart when growing on pumice; bearing pale brown or chestnut-brown non-glandular hairs up to 3 mm long. Fronds 220–2450 mm long. Stipes 70–1400 mm long, 2–15 mm diameter, red-brown proximally, chestnut-brown or yellow-brown distally, with two dark vertical bands (when fresh), bearing red-brown non-glandular hairs proximally and colourless glandular and non-glandular hairs distally, up to 5 mm long. Rachises chestnut-brown or yellow-brown proximally, green distally, bearing abundant colourless glandular and non-glandular hairs up to 3 mm long. Laminae 3-pinnate to deeply 4-pinnate-pinnatifid or almost 5-pinnate, ovate or broadly ovate or broadly elliptic, tapering to a pinnatifid apex, 150–1550 mm long, 90–1300 mm wide, light green on both surfaces, herbaceous; colourless glandular and non-glandular hairs on abaxial lamina surface and costae but absent from lamina margin, 0.3–1.5 mm long. Primary pinnae in 15–30 pairs below pinnatifid apex, overlapping, narrowly winged distally, the proximal pair arising at 30–50° to rachis; distal primary pinnae ovate or narrowly ovate; proximal primary pinnae ovate; the longest at or near the base, 55–860 mm long, 27–480 mm wide, apices acute or acuminate, bases stalked. Secondary pinnae decreasing in length along primary pinnae to the distal end, winged distally, or throughout in less divided fronds; the longest ovate or narrowly ovate, 17–360 mm long, 7–170 mm wide, apices acute or acuminate, bases stalked. Tertiary pinnae winged throughout; the longest ovate or narrowly ovate, 5–90 mm long, 2–40 mm wide, apices acute or obtuse, bases short-stalked to adnate. Quaternary pinnae narrowly ovate or oblong, 7–16 mm long, 2–6 mm wide, adnate, deeply divided on larger fronds into ultimate segments up to 4 mm long and 2 mm wide. Veins ending in apices of ultimate segments. Sori ± round, protected by distinct reflexed lamina flaps (green at the base, membranous at apex); paraphyses absent. Mean spore size 38–39 μm long, 22–25 μm wide; perispores pale, echinate and reticulate.
Hypolepis dicksonioides is generally the largest of all the New Zealand species (except for stunted fronds found growing on pumice), and can be confused only with H. ambigua, with which it often grows in thermal sites. Hypolepis dicksonioides can be distinguished by its long, colourless, glandular hairs, thicker stipes (5–15 cf. 1.5–6 mm diameter), and well-developed indusial flaps.
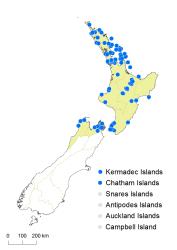
North Island: Northland, Auckland, Volcanic Plateau, Gisborne, Taranaki, Southern North Island.
South Island: Western Nelson, Sounds-Nelson.
Kermadec Islands, Chatham Islands.
Altitudinal range: 0–750 m.
Hypolepis dicksonioides occurs on Raoul, Macauley and South Meyer Islands in the Kermadec Islands. In the North Island it occurs mostly in coastal and lowland sites from Te Kao, Aupori Ecological District, to Auckland, and in scattered localities from the Bay of Plenty to Wellington. It occurs at higher elevations around thermal areas from Rotorua to Taupō, and reaches 750 m near Wairakei. In the South Island it occurs only in coastal areas of north-west Nelson and the Marlborough Sounds. It has also been recorded from the Chatham Islands.
Also Norfolk Island, Samoa, Cook Islands, Tahiti and Marquesas Islands (Brownsey 1987; Sykes 2016). It is naturalised in South Australia (Brownsey 1998).
Grows in open places, on rocky coastal slopes, among boulders, at cliff bases, on pumice beds and scoria (where it is often very stunted), on thermally heated soil and along hot streams, in ditches, on roadsides, in poor pasture, on old logs, on forest margins, in open coastal forest and forest clearings, and under Pinus plantations. It prefers disturbed or sandy soils, and sometimes grows amongst bracken. It also appears as a weed in gardens and urban environments.
Hypolepis dicksonioides was given a conservation status of Naturally Uncommon by de Lange et al. (2018).
There is evidence for hybridisation between H. dicksonioides and H. ambigua (AK 266639, CHR 212707, WELT P011525) and H. lactea (WELT P011537). Hybrids can be recognised by their aborted spores (see Brownsey & Chinnock 1984). The combination H. ambigua × dicksonioides occurs frequently in thermal areas where the two parents are found together.
n = 104 (Brownsey & Chinnock 1984).
In New Zealand this species has been widely misidentified by earlier authors as Hypolepis tenuifolia (G.Forst.) Bernh. ex C.Presl.
Brownsey (1987) noted that Hypolepis dicksonioides has a disjunct distribution, with lowland populations occurring in New Zealand (from sea level, reaching 750 m around thermal areas), the Kermadec Islands (up to 250 m) and Norfolk Island (up to 300 m), and higher-altitude populations on Samoa (1200–1700 m), Tahiti (785–1560 m) and the Marquesas Islands (1020 m). However, populations in Samoa have smaller spores (32–33 μm long, 20–21 μm wide) than those from elsewhere (38–43 μm long, 22–26 μm wide). Whether this is indicative of a difference in chromosome number, and perhaps a different taxon, requires further investigation.