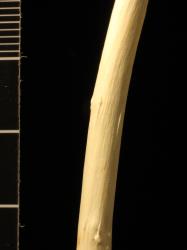
Multi-stemmed shrubs with erect branches, bark grey, smooth. Branchlet wood ridges absent or sparse and short, 3–7 mm long. Current year's branchlets dark red-brown (UCL44). Year-old glabrous, 2.3–2.9 mm diameter. Bud scales 7 mm long, 4.2 mm wide, 3.0 mm deep, ovoid, with two indistinct angles, glabrous, brownish orange (UCL54). Leaves alternate. Stipule in early summer 1.5 mm long, ovate, strongly toothed, hairy, not persisting. Petiole 20 mm long, tomentum of leaf emergence rapidly lost except in leaf axil, glands absent, base of petiole not expanded, green with dark red-brown (UCL44) tinting where exposed to sun. Emerging leaves green, with dense velvety tomentum on lower surface especially on midvein and side-veins, with sparse tomentum on the upper surface, mainly present on the midvein and side-veins, hairs on lower surface silver. Proximal leaves distantly crenate. Leaf lamina 85–120(150) mm long, 53–61(70) mm wide, length to width ratio 1.6–2.1:1, elliptical, slightly asymmetrical at base with one side obtuse, the other rounded; apex acute; leaf galls absent; margins undulate, crenate, tiny teeth displaced to the upper surface, finely revolute; upper lamina surface slightly bullate due to impressed veins, slightly glossy, hairs of leaf emergence rapidly lost, mature leaves glabrous, stomata absent; all veins on the lower lamina surface raised, distinctly glaucous, moderately densely tomentose, hairs all silvery. Catkins female or male, emergence preceding leaves. Flowering branch 28–31 mm long, with 4 reduced leaves. Male catkin 19–30 mm long, 10–28 mm diameter; catkin rachis not visible between flowers. Female catkin 17 mm long, 10 mm diameter; catkin rachis not visible between flowers. Flower bract 2.0–2.8 mm long, 0.6 mm wide, dark brown in upper half, flat, green in the lower half; apex rounded or acute to obtuse; long-silky hairs dense on both surfaces and margins; female bract persisting. Male nectary 1, 0.6 mm long, 0.35 mm wide. Stamens 2, filaments united at the base, hairs absent; anthers 1.0–1.2 mm long, yellow with a slight orange tint before dehiscing. Female nectary 1, 0.4–0.5 mm long, 0.2–0.4 mm wide, pale yellow; ovary 1.6–2.4 mm long, densely short-silky hairy; stipe 0.6–1.9 mm long; style base 0.2 mm long, concealed by ovary hairs, style arms 0.4–0.6 mm long, unlobed, stigmatic surfaces pale yellow-green.
Most similar to Salix atrocinerea, S. cinerea, and the hybrid S. ×reichardtii. Bark is grey and smooth, the erect multi-stemmed trunk is very similar but attaining a greater height than in S. cinerea but similar to that of S. ×reichardtii. Leaves of S. caprea are elliptic with undulate-serrate margins, the upper leaf surface is finely bullate due to impressed veins, the leaf is tomentose above and below, and the lower surface is not glaucous. Catkins are ovate to narrowly ovate, densely long-silky hairy, on a very short branchlet. Flower bracts are dark brown in the distal half, green in the proximal half. The ovary is stalked (0.6–1.6 mm long), covered in short silky hairs, the style base very short and obscured by hairs of the ovary. Style arms are 0.4–0.6 mm long. Stamens 2, filaments are long, without hairs, anthers are yellow and 1.0–1.2 mm long.
Meikle (1984) says Salix caprea branchlet wood lacks ridges. Argus (2010) allows that the wood may have short, sparse wood ridges, and this is confirmed by a diploid plant in the Christchurch Botanic Gardens, which has sparse ridges 3–7 mm long. The presence of short, sparse wood ridges in both S. caprea and S. ×reichardtii makes it difficult to distinguish the two. Both are moderately densely tomentose on the lower leaf surface and sparsely hairy to glabrous on the upper surface of mature leaves. Neither is strongly glaucous on the lower surface, and both have raised netted veins below and impressed veins above. Catkins of both have bicoloured flower bracts that are densely hairy, and anthers of both are yellow. Leaves are elliptical and the length to width ratio is similar (1.6–2.1:1 in S. caprea versus 1.8–2.9:1 in S. ×reichardtii), but leaves of S. caprea are 53–61(70) mm wide compared to those of S. ×reichardtii (22–46 mm wide). Brown hairs are usually present on the upper leaf surface of S. caprea but absent from S. ×reichardtii.
Salix cinerea differs in having peeled wood with dense, long ridges. It also has smaller leaves (lamina 65–103 × 27–44 mm in S. cinerea; 115–120 × 53–61(70) mm in S. caprea). The upper leaf surface has denser tomentum in S. cinerea. The leaves are crenate but not undulate in S. cinerea, while S. caprea has serrate leaves in which the teeth are obscured by the marginal undulations. Young flushing leaves of S. caprea can be very glossy, but are always dull in S. cinerea. Salix caprea has yellow-green, glabrous, 1-year-old branchlets, while S. cinerea has grey-brown branchlets with dense tomentum that persists partially on older branchlets and becomes blackened with fungus.
Salix atrocinerea and S. caprea share brown leaf hairs. The wood ridges of S. caprea are short and sparse or absent, while in S. atrocinerea they are dense and long (averaging 16 mm).
Similar to Salix hookeriana in leaf size and shape and indumentum. The leaf base of New Zealand clones of S. hookeriana is cordate or at least truncate, while in S. caprea it is obtuse but never truncate or cordate. Salix hookeriana lacks visible stipules, while in S. caprea they are obvious and persistent.
Northland (Waiwhatawhata Stream 2009), Southern North Island (Kimbolton 1928), Canterbury (Deans Bush 1954, Ashburton River 1964). The species appears to be uncommon in cultivation.
Allan (1940) says "1925, Cheeseman. Established along streams and in damp places in several localities, especially in North Island." Sykes (1988, p. 1168) said that "Although S. caprea itself, goat willow, is sometimes planted and has been recorded wild in N. Z. many times during the past century, all such records refer to S. cinerea or S. ×reichardtii." However, there are a few wild records; e.g. P.J. de Lange & J.R. Rolfe, 23 October 2009, Northland, Waiwhatawhata Stream, near Ōmāpere, "common along northern riverbank near road", AK 307601; A.J. Healy & B. Parham 64/49, 29 February 1964, Ashburton River, south side "wasteland", CHR 188309.
First collection: Roadside between Kimbolton and Rangiwahia, V.D. Zotov, 5 November 1928, CHR 208.
First publication: Salix caprea was commonly offered for sale in newspaper advertisements by 1925 (e.g. Christchurch Press 1925 p. 16). Healy (1968) listed S. caprea and S. cinerea as adventive in Canterbury but did not list the hybrid S. ×reichardtii. As suggested by Sykes (1988), these records of S. caprea are probably S. ×reichardtii.
Flowering: Mid-September–early October (Newstrom-Lloyd et al. 2015).
Diploid, 2n = 38 (CCDB based on 22 counts). A diploid species in contrast to S. cinerea and S. atrocinerea and the hybrid S. ×reichardtii, which are tetraploid. Flow cytometry of CHR 639166 from Christchurch Botanic Gardens (labelled as S. caprea 'Pendula') indicates this plant is diploid. Flow cytometry indicates two of three clones at Aokautere (PN309 and PN233) are provisionally tetraploid but morphologically are best placed in S. caprea.