- ≡ Zygodon cyathicarpus Mont., Ann. Sci. Nat., Bot. sér. 3, 4: 106 (1845)
- = Zygodon integrifolius Beckett, Trans. & Proc. New Zealand Inst. 25: 297 (1893)
- = Zygodon compactus Müll.Hal., Hedwigia 37: 134 (1898) nom. illeg.
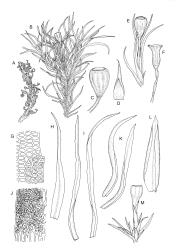
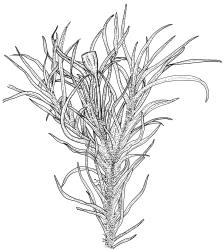
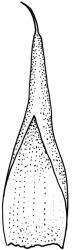
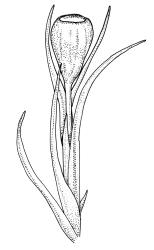
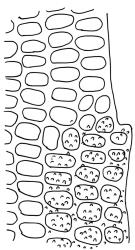
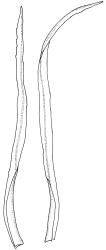
Plants small to medium-sized, yellow-green above, brown below. Stems commonly 15–25(–35) mm in N.Z. material, in cross-section c. 8 cells across, otherwise as per genus. Leaves erect-spreading when moist, strongly contorted when dry, linear-lanceolate and narrowly acute, usually weakly and rather bluntly toothed or less often undulate to nearly entire, plane at margins, mostly 2.5–3.0(–4.0) mm; upper laminal cells oblate or subquadrate, rounded, incrassate, mostly 9–12 µm in greater dimension, with 8 or more small round papillae over the lumen and the contiguous cell walls; papillae apparently becoming elliptic and non-pigmented towards the insertion and in the juxtacostal cells (see discussion under the genus); basal cells pale or hyaline, becoming gradually more elongate-rectangular, thinner-walled, and less papillose-striolate towards insertion; alar and marginal cells not differentiated. Costa strong and subpercurrent, the abaxial surface with elongate and smooth superficial cells nearly to the apex, the adaxial surface with ± quadrate and papillose superficial cells, with a single layer of median guide cells.
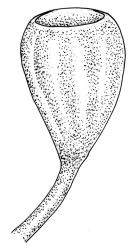
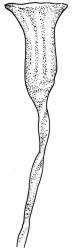
Autoicous. Perichaetial leaves not or scarcely differentiated from the vegetative leaves, linear-lanceolate, neither tubulose nor sheathing the setae, the longest usually exceeding the mouth of the capsule, (2.0–)2.5–3.8 mm. Perigonia on short bud-like branches immediately below the perichaetium. Setae 1 or occasionally 2 per perichaetium, (1.0–)1.7–2.1 mm (excluding vaginula), straight or flexuose; capsules immersed or emergent relative to the inner perichaetial leaves, c. 1.0 × 0.6 mm, otherwise as per genus; exothecial cells, stomata, annulus, and operculum as per genus. Peristome absent. Calyptra as per genus. Spores 11–15 µm.
Beckett 1893, pl. 37 (as Zygodon integrifolius); Magill & van Rooy 1998, fig. 136, 1–12; Malcolm & Malcolm 2003, p. 2.
Amphidium cyathicarpum is best distinguished from A. lapponicum by the features in the key (above); in my opinion sterile material cannot be reliably distinguished from its congener despite the tendency for the vegetative leaves of the present species to become weakly toothed.
Amphidium cyathicarpum can also be confused with other epilithic acrocarps, especially Dicranoweisia antarctica, Anoectangium bellii, Gymnostomum calcareum, and even some Zygodon spp. Sporophytic characters readily distinguish A. cyathicarpum from the also autoicous Dicranoweisia antarctica. The leaves of Amphidium are generally broader in appearance and less strongly cork-screwed when dry, but this difference is subtle and microscopic leaf features are more reliable. The upper laminal cells in Amphidium are subquadrate to oblate and very densely and obviously papillose. In Dicranoweisia the upper laminal cells are generally longer (to 3:1), not noticeably papillose, but bear very fine and often faint linear striations, which sometimes require staining to be observed. The alar cells in Amphidium are not differentiated, while in Dicranoweisia they form a well-differentiated group. Amphidium generally grows on more cation-rich rock types than the Dicranoweisia, although the two species can occur in close proximity.
Amphidium cyathicarpum is a more robust plant with generally longer leaves (2.5–4 mm vs c. 1.5–2.0 mm) than Anoectangium bellii. In cross-section the costal guide cells in A. cyathicarpum are enclosed by abaxial and adaxial substereids, while in Anoectangium the guide cells are exposed on the adaxial surface. In addition, Anoectangium bellii is dioicous and has sheathing perichaetial leaves and a stem central strand.
Compared to Gymnostomum calcareum (with which it often occurs), A. cyathicarpum is a more robust and browner plant (G. calcareum is usually a bright yellow-green plant when fresh). The leaves of A. cyathicarpum are longer (c. 2.5–4.0 mm), less lingulate, and more acute apically than those of Gymnostomum, which are shorter (c. 1 mm), decidedly lingulate, and often obtuse apically. The costa in Amphidium usually occupies c. ⅕ the width of the leaf base and is subpercurrent, while that of Gymnostomum is relatively stouter (c. ⅓ the leaf base) and ends several cells beneath the leaf apex. Sporophyte characters also serve to clearly differentiate these two genera.
Species of Amphidium could be confused (and sometimes grow together) with Zygodon species with acute leaves, such as Z. intermedius. The longer, narrower, and more crisped leaves together with the large area of pale basal cells in Amphidium should preclude confusion with any Zygodon. Also, the laminal cells of Amphidium tend to be oblate and have low and unbranched papillae, which are not restricted to the cell lumen, in contrast to the relatively high and branched papillae over the cell lumen in some species of Zygodon. When fruiting, the immersed to emergent, ± erect, and urceolate capsules borne on short setae in the N.Z. species of Amphidium contrast with the exserted ellipsoid capsules borne on elongate setae in all Zygodon species.
NI: Gisborne (East Cape, Mt Whanakao, Lake Waikaremoana), Taranaki (Dawson Falls), Wellington (Otūpae near Taihape, near Martinborough); SI: Nelson, Marlborough, Canterbury, Westland (Ōtira River), Otago.
Apparently Austral-Andean. The records of Magill & van Rooy (1998) of A. tortuosum (Hornsch.) Cufod. are likely this species. They record A. tortuosum from a wide range of southern hemisphere localities, including Australasian, and north to Mexico.
On rock faces and sides of boulders in stream gorges, waterfall margins, and other sheltered sites; occurring on a wide range of rock types including greywacke, limestone, basalt, papa, gneiss, and sandstone. Ranging on the SI from near sea level (Akatore Stream, Otago L.D.) to at least 1500 m (Temple Basin, Canterbury L.D.). It is frequently associated with Zygodon intermedius, Bartramia papillata, Fissidens leptocladus, Austrohondaella limata, Tortella knightii, and Gymnostomum calcareum. Collections suggest that it is far more common on the South I. than on the North I. There are at least 20 independent collections in CHR herbarium from Canterbury L.D., and more than 10 collections from Otago L.D. Only a single Westland collection, from c. 760 m in the Ōtira Gorge, has been seen.
The present species seems to differ from the northern hemisphere A. mougeotii primarily by its autoicous sexuality and by its southern distribution. The placement of A. cyathicarpum in synonymy with the South African A. tortuosum (Hornsch.) Cufod. by Magill & van Rooy (1998) is not followed here, in part because they were unable to sight type material of the latter name. Under the circumstances it is preferable to continue use of the well-known name A. cyathicarpum, based on a Chilean type. The full resolution of both these taxonomic and nomenclatural questions exceeds the scope of this Flora.