- ≡ Cladomnion pallens Sande Lac., Sp. Nov. Musci Ind. 12 (1872)
The species description below deliberately repeats information in the generic description, reflecting my persistent doubts about a close relationship between these two species.
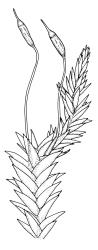
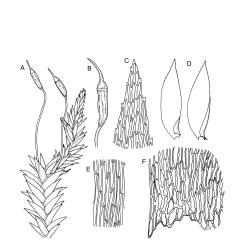
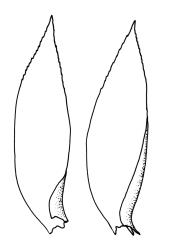
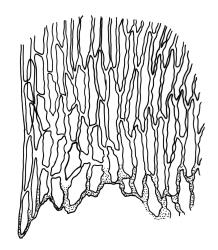
Plants yellow- or pale green, strongly lustrous, moderately robust, forming upright tufts. Primary stems weak, apparently forming thin and creeping mats. Secondary stems (shoots) in tufts, c. 8–23 mm, simple or branched, curved, strongly complanate, tapered to a pointed tip, with stems oval in cross-section, lacking a central strand, with several layers of thick-walled, pigmented cortical cells, and with a rather dense mat of ± smooth brown rhizoids at base. Leaves of secondary shoots arranged in 4 rows, strongly complanate, imbricate, not decurrent, asymmetrically lanceolate, tapered to an acute apex, not plicate, flat, margins strongly incurved at extreme base at one side but otherwise plane, serrulate in upper third, mostly c. 2.0–2.5 × 0.5–0.7 mm, becoming smaller towards shoot apices, sometimes with leaves of branches narrower (c. 0.3 mm wide) and nearly symmetric; mid laminal cells linear-rhombic, thin-walled, not porose, smooth, mostly 90–105(–120) × 4–5 μm, becoming shorter but not otherwise differentiated towards apices; cells at insertion shorter, thicker-walled, not porose, weakly pigmented but scarcely forming a distinct band; alar cells weakly differentiated, a few ± quadrate and thick-walled in extreme angles. Costa absent or very short (<⅕ of leaf), double, and indistinct. Paraphyllia absent. Gemmae present among upper leaf axils, acicular, uniseriate, mostly 12–18 cells and 400–650 μm long, arising in tufts of c. 30–50 from short rhizoid-like structures.
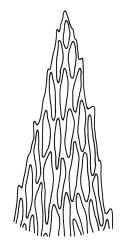
Probably dioicous. Perichaetia scattered on secondary shoots, the leaves apparently expanding greatly after fertilisation, the inner leaves ovate-lanceolate, c. 1.2 mm, clasping the seta base. Male plants and perigonia not seen. Setae 6–8 mm; capsules erect, symmetric, ellipsoid-cylindric, strongly 8-ribbed when dry, c. 2 mm; exothecial cells firm-walled, rounded, and ± thickened at corners; stomata few at extreme capsule base; annulus differentiated, of c. 3 rows of inflated cells, persistent; operculum as per genus, c. ½ to nearly equal the length of the capsule. Exostome teeth yellow-brown, lanceolate but ± shouldered above, c. 380 μm, bordered, inserted near the mouth, cross-striate and furrowed c. ⅞ to apex on outer surface, densely trabeculate but otherwise nearly smooth on inner surface; endostome with a pale membrane c. ½ the height of the teeth, segments smooth, nearly the height of the teeth. Calyptra not seen, probably cucullate and smooth. Spores ellipsoid or round, mostly 24–30 μm, finely papillose.
Brotherus 1925, fig. 510; Sainsbury 1955, pl. 54, fig. 3; Enroth 1991, fig. 1, a–f.; Milne & Klazenga 2012, unnumbered fig.
NI: S Auckland (between Lake Rotoehu and coast, Wairere Stream in Kaimāī Range, Mayor I.).
Anomalous. Reported from New Guinea (both P.N.G. & West Irian) and Taiwan by Enroth 1991) and from mainland Australia (Qld, N.S.W., and Vic.) by Milne & Klazenga (2012).
Documented as growing on Ripogonum and Rubus stems, on small branches and twigs of unspecified trees, and on a fallen dead branch in tawa/kāmahi/tāwari forest. Apparently from c. 180–400 m elevation.
This is one of N.Z.’s rarest and most poorly documented moss species. Five K.W. Allison collections made between December 1940 and March 1943 are from the area between Lake Rotoehu and the Bay of Plenty coast. Most of Allison’s collections were made from Ripogonum and lawyer (presumably Rubus cissoides) stems at c. 180 m elevation and appear to come from moist gullies. One collection (K.W. Allison 644, CHR 547290B, WELT M003012) is better documented than the others and came from "lawyer vines of about 1 inch diameter [in a] broad bush gully, open underneath". Another collection (K.W. Allison 645, CHR 486029; WELT M003013) bears slightly more detailed locality data than the others and was collected at "northern part of bush on Hannon’s Road". The region north of Lake Rotoehu is presently largely occupied by exotic pine plantations and it is unclear if H. pallens survives in this area.
More recent collections from the Kaimāī Range (M. Skinner s.n., CHR 352758) and Mayor I. (P. de Lange 10375, AK 330304) extend the known range of this rare species westward and northward but all known localities remain in S Auckland L.D. This species is given a Nationally Critical ranking, with the modifiers of Data Poor and Range Restricted in the 2014 iteration of the Conservation Status of N.Z. (Rolfe et al. 2016).
Scott & Stone (1976, p. 361), describe the shoots as curving "outwards and upwards like hooks". I have been unable to locate perigonia and doubt Sainsbury’s (1955p. 344) statement that H. pallens is autoicous. Brotherus (1925, p. 103) states the genus Hampeella to be dioicous, and Enroth (1991) stated unequivocally that this species is dioicous in P.N.G. He described perigonia from there, and also briefly described the habitat and substrate of this species in P.N.G.
When leaves of this species are removed, a few stem cells often adhere to the leaf base, giving the incorrect impression of decurrencies. It is difficult to make consistent observations on spores in herbarium material. Although in most of the capsules examined (e.g., in K.W. Allison 2055 and 2054) the spores are as described above, in one capsule of K.W. Allison 2055 the spores appear to be dimorphic, with many spores smaller and apparently collapsed.
Material has been seen (K.W. Allison 644, CHR 547290A & B) in which the leaves of some upper branches are decidedly more symmetric and narrower (c. 0.3 mm wide) than the leaves of the lower shoots. At least one duplicate of K.W. Allison 644 (WELT M003012), as well as other collections from the same locality, were annotated by R. Hattaway (who wrote a Ph.D. dissertation on this family) in 1980 as H. pallens var. symmetrica, but these specimens fall within the normal range of variability of H. pallens. The status of H. pallens var. symmetrica, has been further confused by the fact that both Sainsbury and Hattaway (in herb.; see also Sainsbury 1951) erroneously annotated material of both representative H. pallens and H. alaris as H. pallens var. symmetrica. The holotype of var. symmetrica (K.W. Allison 2052, WELT M003017) is, however, clearly referable to H. alaris. The var. symmetrica is neither accepted nor discussed further here.
The peristome of H. pallens is strongly suggestive of species in the Daltoniaceae.