- ≡ Mniopsis plumula Mitt. in Wilson, Bot. Antarct. Voy. III. (Fl. Tasman.) Part II, 187 (1859)
- = Mniopsis rotundifolia Müll.Hal., Hedwigia 36: 332 (1897) nom. illeg.
- ≡ Mittenia rotundifolia (Müll.Hal.) Paris, Index Bryol. Suppl. 248 (1900)
Elements in the following description are taken from Stone (2006).
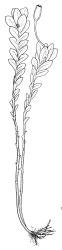
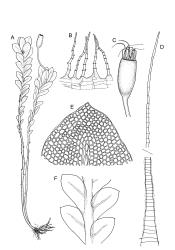
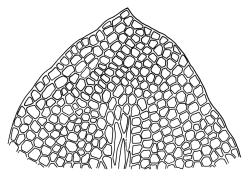
Plants grass green to dark green, rarely red-brown, gregarious or occurring as scattered shoots, often forming dense turves on deeply shaded friable soil. Stems simple, often several clustered, mostly c. 10 to at least 20 mm, green above and grading to dark red-brown near base, in cross-section weakly angled (± pentagonal) and with a faint central strand, with elongate, smooth, and often brittle yellow-brown rhizoids near base. Protonema sometimes persistent, with highly refractive lenticular cells and gemmae. Leaves distichous, scale-like below, becoming larger and evenly but distantly spaced above, rarely well-developed only at shoot apex, vertically inserted, oblong and tapered to an obtuse apex, entire, plane, with the proximal margin strongly and broadly decurrent nearly to the leaf below (but not confluent), mostly 0.6–1.0 mm on the distal margin and longer (mostly 0.9–1.3 mm) on the proximal margin, c. 0.40–0.55 mm wide; upper laminal cells mostly quadrate to short-rhombic, firm-walled, smooth, the lumina densely packed with chloroplasts, mostly 15–24 µm in greater diameter, becoming more irregular in outline below. Costa stout, but ill-defined under compound microscope, 45–60 µm wide at mid leaf and failing c. 6–10 cells below apex, in cross-section elliptic and scarcely projecting, with a few central stereids and larger cells on both the abaxial and adaxial surfaces.
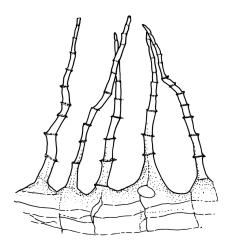
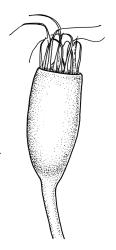
Dioicous. Perichaetia terminal, the perichaetial leaves radially arranged, transversely inserted and lacking decurrencies, enlarged, c. 1.4–1.8 × 0.55 mm, surrounding c. 12–15 archegonia and apparently lacking paraphyses. Perigonia not seen in N.Z. material. Setae single or occasionally paired, rather delicate, 2–4 mm, nearly hyaline to green at capsule maturity; capsules cylindric and symmetric, erect, slightly flared at mouth, variable in size, mostly c. 0.7–1.0 × 0.35–0.4 mm diam. (occasionally as short as 0.5 mm), pale brown, red-brown at mouth; exothecial cells oblong, with firm longitudinal walls and thin transverse walls; stomata difficult to observe, superficial and few at base of the capsule; annulus absent; operculum long and obliquely rostrate from a conic base, nearly the length of the urn. Peristome double; exostome teeth 16, red-brown, very long and slender, recurved at base and strongly inwardly twisted above when dry, erect at base and strongly curved-reflexed above when moist, mostly c. 600–700 µm (difficult to measure due to curvature), the outer surface apparently nearly smooth and with c. 13 transverse ridges, the inner surface with numerous (c. 100) lamellae, which give the teeth a densely articulated appearance, lacking a divisural line or surface ornamentation; endostome composed of 16 nodose but otherwise smooth segments and arising from a very low basal membrane, lacking cilia. Spores 9–12, pale, very finely ornamented.
Brotherus 1924, fig. 373; Stone 1961b, figs 1–20, pls xx–xxii; Stone 1961a, figs 1–81, pls 1–4; Shaw 1985, figs 1–10; Malcolm & Malcolm 2003, p. 44; Meagher & Fuhrer 2003, p. 45; Malcolm & Malcolm 2006, pp. 71, 79, 87, 137.
The placement of Mniopsis rotundifolia Müll.Hal.in synonymy follows Dixon & Bartram (1937, p. 77). Stone (2006) also adopted the synonymy advocated by Dixon & Bartram; she also was unable to view type material but indicated her belief that material was present in the Brotherus herbarium.
Mittenia is most likely to be confused with species of "micro" Fissidens, because they share a strongly distichous habitat and because many species of Fissidens share its preference for deeply shaded soil banks. Mittenia lacks the vaginant lamina and the strongly conduplicate "dual laminae" leaf structure found in all species of Fissidens. The leaves in Mittenia are generally widely spaced on the stems and always strongly decurrent, while the leaves in "micro" Fissidens species are generally ± imbricate and never strongly decurrent.
NI: N Auckland including offshore islands (LB), S Auckland, Gisborne (Waioeka Gorge), Taranaki, Wellington; SI: Nelson, Marlborough (Ship Cove Scenic Reserve), Canterbury, Westland, Otago (Cardrona, Leith Valley), Southland; St; Ch.
Australasian. Mainland eastern Australia*. Recorded also from Tasmania and from one locality in Western Australia by Stone (2006) and from a single collection in P.N.G. by Norris & Koponen (1987).
Widespread through most of N.Z., on deeply shaded and friable soil and often associated with the root plates of wind-thrown trees. Also on shaded and often eroded banks, beneath overhanging vegetation, in "earth-caves", soil crevices, and cave or mine entrances. Not occurring directly on rock and avoiding limestone; sometimes forming extensive and dense turves or swards. Occurring in a wide range of vegetation types, including mixed broadleaved and southern beech-dominated forests. It is poorly documented and probably very uncommon in both Marlborough and Otago L.D.; it is not recorded from Hawke’s Bay L.D. At Puketoki Scenic Reserve, near Whakamārama (S Auckland L.D.) fruiting plants of M. plumula have been observed forming a nearly pure sward as much as 20–30 cm wide and many metres in (horizontal) length on a deeply shaded, ± vertical, track-side silt bank derived from volcanic bedrock in a Beilschmiedia tawa-dominated lowland forest. Ditrichum spp. (especially D. difficile ) are probably the most frequently associated mosses but other often associated bryophytes include Fissidens asplenioides, F. pallidus, F. tenellus, Leucobryum javense, Rhizogonium pennatum, the hepatics Balantiopsis spp., Kurzia hippuroides, Lepidozia spp., Tylimanthus diversifolius, and Zoopsis argentea as well as the lichen genus Lepraria. On North I. occurring from c. 80 m (Whanganui River, Taranaki L.D.) to at least 750 m (Erua and the Akatarawa Ranges, both Wellington L.D.) and on South I. from c. 30 m (Pororari River, Nelson L.D.) to 1150 m (Paparoa Range, Nelson L.D.).
Stone recorded gemmae to be formed on both the protonema (Stone 1961b) and on rhizoids (Stone 1961a). No gemmae have been seen in N.Z. material.
The "luminescence" for which M. plumula is renowned is very striking but is relatively rarely seen, at least in N.Z. Stone (2006) described colonies of luminous protonema in Australia that "sometimes covers very large areas and can persist for many years, reproducing asexually and forming few or no sterile gametophores". Such growth may be a response to marginal growth conditions.