- ≡ Hypnum purpurascens Brid., Muscol. Recent. Suppl. 2, 121 (1812)
- = Anictangium humboldtii Hook., Pl. Crypt. pl. 1A (1816)
- ≡ Hedwigia humboldtii (Hook.) Hook., Musci Exot. 2, 137 (1819)
- ≡ Rhacocarpus humboldtii (Hook.) Lindb., Öfvers. Kongl. Vetensk.-Akad. Förh. 19: 607 (1863)
- = Anoectangium humboldtii var. australe Hook.f. & Wilson, Bot. Antarct. Voy. I. (Fl. Antarct.) Part I, 135 (1845) – as var. β australe
- ≡ Hedwigia humboldtii var. australis (Hook.f. & Wilson) Hook.f. & Wilson in Wilson, Bot. Antarct. Voy. II (Fl. Nov.-Zel.) Part II, 93 (1854)
- = Harrisonia strictipila Müll.Hal., Österr. Bot. Z. 47: 394 (1897)
- ≡ Rhacocarpus strictipilus (Müll.Hal.) Paris, Index Bryol. Suppl. 293 (1900)
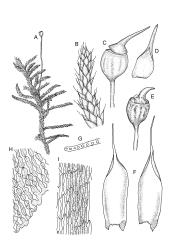
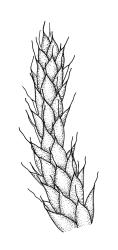
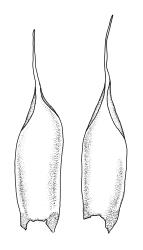
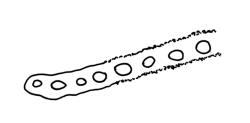
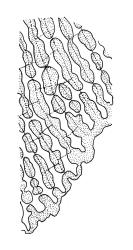
Plants lustrous and appearing lacquered when dry, yellow-green to castaneous below, usually glaucous in parts and with bright castaneous or golden hair-points, rarely black throughout, forming prostrate and loosely interwoven mats or self-supporting and sub-erect, subpinnately branched. Stems (20–)60–80(–100 mm or greater). Rhizoids apparently absent. Stem and branch leaves differentiated. Stem leaves erect, imbricate both moist and dry, inserted in ranks and sometimes appearing funiculate, with an oblong-panduriform base and appearing abruptly tapered (due to inrolled upper margins) to a very slender, pale, golden, or castaneous hair-point, ecostate, concave, smooth even when dry, strongly inrolled at upper margins (and thus accentuating the taper of the leaf tip), denticulate near the base of the hair-point, entire below, auriculate and decurrent at insertion, mostly c. (2.0–)2.3–2.9 × (0.6–)0.8–1.0 mm (under cover slip). Branches mostly 7–10 mm, cuspidate at apex. Branch leaves mostly c. ⅔ the length and width and less strongly decurrent than stem leaves; upper laminal cells as per genus, mostly 27–42 × 7–9 µm; basal cells as per genus; marginal cells forming a distinct and often pigmented border c. 3–4 cells wide (at mid leaf) and extending from the leaf base to mid leaf or nearly to the base of the hair-point; alar cells rectangular to quadrate, extremely thick-walled and porose, forming a conspicuous, strongly pigmented, and auriculate group.
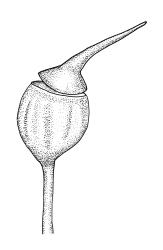
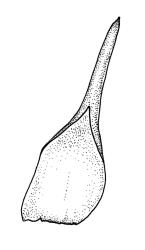
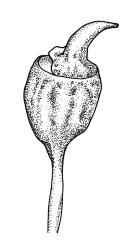
Dioicous. Perichaetia occurring in clusters of 2–4 or more, at intervals (apparently at annual growth intervals) on stems, the inner leaves oblong-lanceolate, strongly pigmented, c. 4 mm long, and strongly sheathing the seta base, with laminal cells lacking cuticular reticulations. Perigonia inconspicuous, best observed near stem apices, c. 2 mm, the bracts very concave, more ovate and pigmented than vegetative leaves, surrounding antheridia and numerous filiform 5–6-celled paraphyses. Setae c. 10–20 mm, red-brown, weakly dextrorse; capsules erect, broadly obovoid with a poorly defined neck, weakly furrowed when dry, 2.0–2.5 mm, nearly globose after dehiscence; exothecial cells mostly isodiametric and firm-walled, with ill-defined vertical bands of thinner-walled cells, becoming much smaller near mouth; stomata immersed, few and restricted to neck; annulus lacking; operculum obliquely rostrate, ± equal the capsule in length. Spores ± tetrahedral, 21–28 µm, coarsely papillose-lirate.
The habitat drawing, fig. A, inadequately illustrates the conspicuous hair-points. Scott & Stone 1976, p. 359; Beever et al. 1992, fig. 54; Crum 1994, fig. 498; Magill & van Rooy 1998, fig. 151, 1–11; Malcolm & Malcolm 2003, p. 58; Meagher & Fuhrer 2003, p. 77; Seppelt 2004, fig. 96; Seppelt et al. 2013, pl. 26.
Some material of R. purpurascens could conceivably be confused with alpine forms of Wijkia extenuata var. extenuata given their similar growth habit, branching patterns, and abruptly tapered leaves. Rhacocarpus purpurascens differs from the Wijkia by having leaves that appear thick and lacquered with pigmented hair-points. The alar cells of R. purpurascens are rectangular, extremely thick-walled, strongly pigmented, and porose, in contrast to the highly inflated and very thin-walled alar cells of the Wijkia. The capsules of the two plants are completely different. Rhacocarpus purpurascens usually grows on rock, while the Wijkia grows mostly on rotten wood.
NI: N Auckland, including offshore islands (GB), S Auckland, Gisborne (Raukūmara Range, Te Rangaakapua), Hawke’s Bay (Black Birch Range), Wellington, Taranaki; SI: Nelson, Marlborough (Mt Stokes, Richmond Range), Canterbury, Westland, Otago, Southland (Longwood Range, many localities in Fiordland); St; A; C; M.
Anomalous. Tasmania*, mainland Australia*, Chile*, Argentina*, Bolivia*, Ecuador*, Dominican Republic*, Rwanda*, Kenya*. Reported also from Mexico, Central America, and from other South American and African localities by Crum (1994). The genus occurs in Malesia, including New Guinea, but the specific status of this material is debatable (cf. Frahm 1996; Koponen & Norris 1986).
An easily recognised species of dripping rock faces (basalt, granite, greywacke, sandstone, serpentine, conglomerate), where it forms extensive prostrate or self-supporting and sub-erect mats, sometimes several square metres in extent. It is nearly ubiquitous in N.Z. and is known from many localities from all the L.D. for which specific localities are not cited above. This species avoids calcareous and/or cation-rich rocks and at lower elevations it prefers south-facing and moist slopes. Also occurring on waterlogged peat, at the margins of intermittent pools in cushion bogs and pākihi, in bryophyte mats over gravel banks, in intermittent streams, and rarely over rotten wood. On the North I. from near sea-level (North Cape, N Auckland L.D.) to at least 1700 m (Mt Hikurangi, Gisborne L.D.), but most common above c. 400 m. On the South I. from near sea level (Dusky Sound, Southland L.D.) to at least 1975 m (Remarkable Range, Otago L.D.) elevation. Frequently associated species in moist sites include Andreaea nitida, Breutelia elongata, B. pendula, Campylopus bicolor, Pulchrinodus inflatus, Racomitrium crispulum s.l., and Warnstorfia fluitans, as well as Isotachis spp., Jamesoniella colorata, Frullania rostrata, Drosera arcturi, and D. spathulata. In drier sites associates may include Andreaea subulata, Austrohondaella limata, Campylopus clavatus, C. introflexus, Dicranoweisia antarctica, Ditrichum punctulatum, and Racomitrium pruinosum, as well as Cladia aggregata and Hypogymnia lugubris.
Once the characteristic appearance of R. purpurascens is recognised, especially the pinnately branched stems, the lacquered and usually glaucous appearance of the leaves with castaneous leaf margins and hair-points (especially in lower portions of the plants), together with the large and strongly pigmented alar group, there is nothing else in the N.Z. flora with which this species could be confused. The fine structure of the leaf surface is also highly distinctive. The degree of hair-point development is highly variable and does not appear to be geographically correlated.
Very strongly pigmented and sometimes nearly black material seems to be associated with prolonged submersion; very dark material frequently has particularly obviously porose laminal cells. Occasional herbarium specimens turn dull grey with age.
Frahm (1996) searched unsuccessfully for type material of Hypnum purpurascens Brid. in the Bridel herbarium and suggested that the selection of a neotype from Réunion might be required.
Both the names Rhacocarpus humboldtii and R. australis have been widely applied in N.Z. literature and herbaria. Rhacocarpus australis (Hampe) Paris [Ind. Bryol.1068, 1898] is founded on Harrisonia australis Hampe [Linnaea 30: 636, 1860], which has a type (not seen) from the Grampian Range of Victoria. Streimann & Klazenga (2002) include H. australis in the synonymy of R. purpurascens and no useful purpose would be served by questioning this placement. Frahm (1996, p. 56) placed Anoectangium humboldtii var. australe Hook.f. & Wilson in the synonymy of R. purpurascens, citing a Victorian specimen gathered by Walter as the type. However, the protologue shows the syntypes of this name are clearly from Auckland and Campbell Islands, and were presumably collected by Hooker. These have not been seen.