- ≡ Dicranum tasmanicum (Hook.f.) Wilson, Bot. Antarct. Voy. III. (Fl. Tasman.) Part II, 171 (1859) – as Dicranum Tasmanicum
- = Dicranum lancifolium R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 458 (1897)
- ≡ Weissia weymouthii var. lancifolia (R.Br.bis) Dixon, Bull. New Zealand Inst. 3: 115 (1923)
- ≡ Weissia lancifolia (R.Br.bis) Wijk & Margad., Taxon 9: 52 (1960)
- = Dicranum rostratum R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 458 (1897)
- = Grimmia beckettiana Müll.Hal., Hedwigia 37: 163 (1898)
- = Cinclidotus australis Dixon, Bull. Torrey Bot. Club 42: 96 (1915)
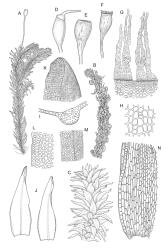
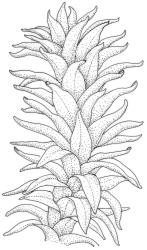
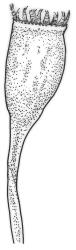
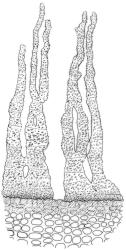
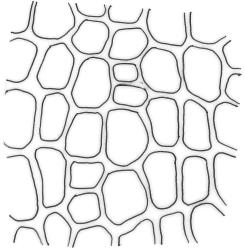
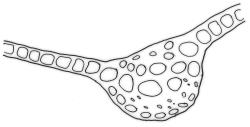
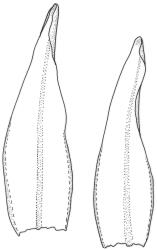
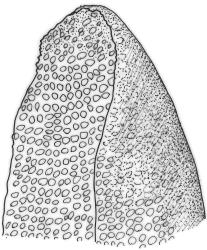
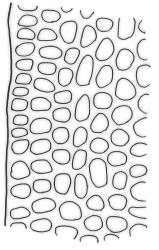
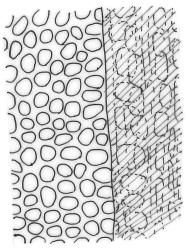
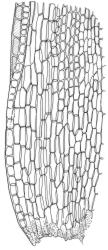
Plants medium-sized to robust, yellow-brown to dark olive-green above, often darker (sometimes nearly black) in lower portions and occasionally throughout, forming rather loose and often extensive turves on wet rocks. Stems variable in length, (5–)15–80 mm, moderately branched, with lower leaves often eroded, sparsely beset with smooth, red-brown rhizoids; in cross-section with or without a central strand, with several layers of thick-walled red-brown outer cells, and an outermost layer with ± thinner walls. Leaves erect-spreading and often weakly spiralled around the stem when moist, strongly incurved and sometimes also cork-screwed when dry, c. (2.5–)3.0–3.5(–5.0) × (0.65–)0.8–1.0 mm, lingulate to lingulate-lanceolate, broadly channelled, rounded and cucullate at apex; margins narrowly reflexed below, plane above, entire; upper laminal cells clear in outline, oval, elliptic to nearly round, mostly 6–9(–12) µm in greater diameter, very firm-walled, smooth, becoming gradually more quadrate and then rectangular towards the leaf base; basal marginal cells strongly differentiated in c. 5 rows, elongate (mostly c. 40–100 µm) and to 10:1, thick-walled, and often strongly red-brown pigmented, usually forming an intra-marginal border extending ⅓ to ½ the leaf length, the outermost row of cells short-rectangular at extreme base, becoming progressively more quadrate and then oblate upwards, merging gradually with the upper laminal cells. Costa strong and sharply defined, failing 5–10 cells before apex, c. 90–110 µm wide in lower leaf; adaxial superficial cells elongate; abaxial superficial cells quadrate distally. Axillary hairs of 3–5 cells, basal cell brown. Laminal KOH colour reaction yellow.
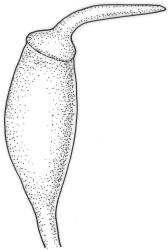
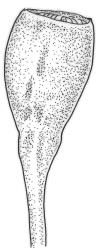
Dioicous. Perichaetia terminal and overtopped by successive lateral innovations, the perichaetial leaves slightly more elongate but otherwise not differentiated. Perigonia inconspicuous, terminal (sometimes overtopped by innovations and then appearing lateral), the inner bracts broadly ovate and c. 1.5 mm, surrounding c. 25 antheridia and numerous filiform, ± hyaline paraphyses. Setae 15–20 mm, smooth, dark red. Capsules oblong-cylindric or turbinate, with a weakly defined neck, smooth or weakly wrinkled when dry, red- or yellow-brown, scarcely constricted below mouth when dry, 1.5–2.0 mm; exothecial cells firm-walled, mostly oblong-ellipsoid; stomata restricted to neck, superficial. Operculum rostrate from conic base, c. 1.3–1.5 mm, sometimes weakly adherent to the columella. Peristome teeth 16, erect when dry, inserted close to rim, broadly lanceolate or ± irregular in outline, pale yellow-brown, irregularly perforate or divided 2–3 times to halfway or more (i.e., irregularly trifid), densely and lowly papillose throughout or weakly baculate above, c. 450–500 µm, basal cylinder lacking. Spores (18–)21–30 µm, smooth.
Brotherus 1924, fig. 225; Scott & Stone 1976, p. 36; Beever et al. 1992, fig. 29; Seppelt 2004, fig. 98; Malcolm et al. 2020, p. 648.
Tridontium tasmanicum is by far the most robust species of the genus, with stems to 80 mm or more and leaves 2.5–5.0 mm. It is the only Tridontium found in truly aquatic habitats, where older leaves are often eroded. Leaf apices are usually strongly cucullate, and are rounded with a failing costa as is typical for the genus. When turbinate, as they often are, the capsules are diagnostic. Laminal cells are invariably smooth.
Confusion is possible with Andreaea australis, from which Tridontium tasmanicum differs by its strongly cucullate leaf apex (the apex of A. australis is flatter, with weakly recurved margins), and by the presence of its distinctive basal intramarginal border. The basal marginal cells of A. australis are very compact and ± quadrate. Andreaea australis, while also occupying rocks in streams and seepages, characteristically grows at higher elevations; when capsules are present, confusion will not occur.
Tridontium tasmanicum could be confused with Schistidium rivulare var. subflexifolium, which grows in similar habitats and often has rounded and ± cucullate leaf apices. The latter also has incurved leaf tips when dry, but this feature is less strongly developed than in T. tasmanicum. Upper laminal cells in S. rivulare var. subflexifolium are obscure due to its bistratose upper lamina (vs clear cell outlines and unistratose upper lamina in T. tasmanicum). T. tasmanicum occurs throughout a much wider elevational range (descending almost to sea level) and on a wider variety of rock types than does the predominantly alpine S. rivulare var. subflexifolium. The uncommon moss Hyophila novae-seelandiae can be distinguished by its recurved, flat leaf apices, often with a minute apiculus (vs leaf apices cucullate and entire in Tridontium tasmanicum).
NI: N Auckland, S Auckland, Gisborne, Hawke’s Bay, Taranaki, Wellington; SI: Nelson, Marlborough, Canterbury, Westland, Otago, Southland; Ch; A; C. Recorded from M by Seppelt (2004).
Australasian. Tasmania*, mainland Australia*. Recorded from New Guinea by Eddy (1990).
Tridontium tasmanicum is a hydrophilous or hygrophilous moss. On sand, silt, or rarely on humus over streamside rocks, sometimes associated with waterfalls and less often with lake margin sites subject to wave action. It is often submerged at time of high stream flows, but also occurs on damp, shaded banks. It is particularly frequent and well developed in areas of calcareous bedrock, but its presence on other rock types probably indicates a preference for substrates of relatively high cation availability, rather than a lime requirement per se. Tridontium tasmanicum grows on thin soil over a very wide range of rock types, including limestone, marble, greywacke, sandstone, siltstone, papa, basalt, schist, and concrete, and is tolerant of at least a moderate amount of salt spray. It can also grow on silt-covered wood at stream edges. It often forms extensive and nearly pure turves at river margins. When growing on limestone it can become encrusted with calcium carbonate deposits. Records range on the North I. from near sea level (Whatipū, Auckland L.D.) to at least 1340 m (Tararua Range, Wellington L.D.); on the South I. from near sea level to at least 1650 m (Temple Basin, Canterbury L.D.). Associated species include Bryum laevigatum, Cratoneuropsis relaxa, Dicranella cardotii, D. vaginata, Fissidens asplenioides, F. rigidulus, F. waiensis, Gymnostomum calcareum, Ochiobryum blandum, Philonotis pyriformis, Tridontium cockaynei, and T. novae-zelandiae.
A distinctive microscopic feature is the intramarginal border of elongate cells in the leaf base contrasting with isodiametric or short-rectangular cells closer to the margin. This is often well developed, but sometimes discernible only with the eye of faith. Similarly, a well-developed stem central strand is often visible in cross-sections (e.g., Seppelt 2004, fig. 98), and has been described as “present and strong" (Zander 1993). However, this feature is also variable, and not correlated with intramarginal border development; e.g., a specimen from damp papa rock on a roadside cliff (Wellington L.D., Mākiekie Scenic Reserve, J.E. Beever 113-50a, CHR 678940) has a well-marked intramarginal border in the leaf bases, but no central strand seen in the stem.
Dicranum rostratum R.Br.bis was placed in synonymy of Tridontium tasmanicum by Dixon (1923, p. 122). Type material has not been seen for this study, but the information in the protologue is sufficient for the synonymy to be accepted.