- ≡ Gymnostomum complanatum Hook.f. & Wilson, London J. Bot. 3: 538 (1844)
- = Calomnion laetum Hook.f. & Wilson in Hooker & Wilson, London J. Bot. 3: 97 (1844) nom. illeg.
- = Calomnion brownseyi Vitt & H.A.Mill. in Vitt, Bryologist 98: 346 (1995)
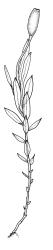
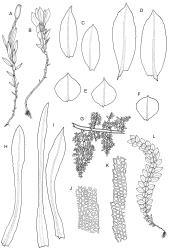
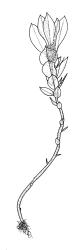
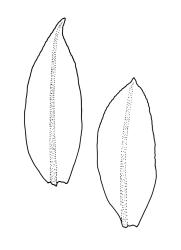
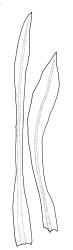
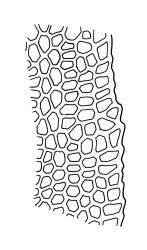
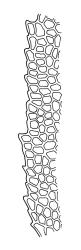
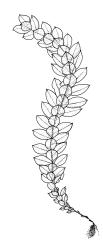
Plants shiny, small, olive-green to light-green or red-brown, mostly on tree-fern caudices. Stems c. 7–12 mm, beset at base with papillose brown rhizoids, often arising from a mass of persistent protonema, sometimes becoming nearly naked because of caducous leaves, in cross-section as per genus. Shoots c. 1.3–1.8 mm wide. Lateral leaves elliptic to ovate-elliptic, acute, bluntly mucronate to short acuminate, entire, crenulate or irregularly denticulate above, asymmetric at base, scarcely altered when dry, mostly 0.7–1.1 mm, but becoming smaller towards stem base; ventral leaves broadly elliptic, acute to weakly acuminate, entire to irregularly toothed; upper laminal cells irregularly polygonal, firm-walled, mostly 6–11 μm in greater diam., unistratose, when dry with the cell contents occasionally contracted to form a primordial utricle; basal cells and alar cells not differentiated. Costa stout, subpercurrent, percurrent, to long-excurrent, often forming a mucro. Protonema persistent, extensive, highly branched, rust-brown, with a rhizome-like, red-brown, and warty caulonema bearing densely bipinnate branches or short, bulging, and chlorophyllose cells.
Dioicous. Perichaetial leaves strongly differentiated, much longer than vegetative leaves, variable in shape, narrowly panduriform, oblanceolate, or narrowly spathulate, acute, acuminate, or rarely obtuse at apex, entire, crenulate, or irregularly denticulate at margins, unistratose throughout or rarely with small bistratose marginal areas, 1.6–2.5 mm, with costa mostly ending a few cells below apex. Perigonia terminal, the inner leaves broadly ovate or panduriform, surrounding up to 15 antheridia and few filiform paraphyses. Setae (2–)3–4.5(–6) mm, slender and flexuose; capsules erect, gymnostomous, ellipsoid or cylindric, (0.8–)1.0–1.4(–1.5) mm, pale brown and ± red at mouth at maturity, weakly sulcate when dry; exothecial cells thin-walled, rounded-oblong or rounded-quadrate; stomata restricted to neck, superficial; annulus well-differentiated, vesicular. Calyptra cucullate, ± scabrous above. Spores 12–14 μm, papillose.
Sainsbury 1955, pl. 43, fig. 3 (as C. laetum); Vitt 1995, figs 8–14; figs 44–47 (as C. brownseyi); Malcolm & Malcolm 2003, p. 10; Catcheside & Bell 2006, pls 54–55.
In a N.Z. context C. complanatum is most likely to be confused with Rhizogonium pennatum or R. novae-hollandiae. While often growing on tree-ferns, both species of Rhizogonium are more robust plants with basal rather than terminal perichaetia. They lack the reduced ventral leaves of C. complanatum; both have stoutly excurrent costae and horizontal to pendent capsules with well-developed peristomes. R. pennatum also has a well-developed leaf marginal border. Aberrant material of C. complanatum could possibly be confused with Leptotheca gaudichaudii, but that species differs by regularly producing dark brown, filamentous axillary gemmae, and having longer (1.6–3.1 vs 0.7–1.1 mm) vegetative leaves with sinuose rather than straight costae. Leptotheca gaudichaudii also lacks reduced ventral leaves and its capsules are longer and peristomate.
K; NI: N Auckland, including offshore islands (GB, LB), S Auckland, Gisborne, Hawke’s Bay, Taranaki, Wellington (including KA); SI: Nelson, Marlborough, Canterbury (Banks Peninsula), Westland, Otago, Southland; St; Ch.
Australasian. Mainland Australia*. Reported from Tasmania by Stone (1990) and Catcheside & Bell (2006). Adventive in Ireland*.
Mainly on tree-fern caudices, commonly on Cyathea smithii, C. dealbata, Dicksonia squarrosa, and rarely on C. medullaris. In N Auckland L.D. also occurring on Blechnum fraseri. Less frequently on trunks of Rhopalostylis sapida (nīkau), Elaeocarpus dentatus or Dracophyllum arborea. Rarely on vertical or overhanging rock faces or vertical clay banks. From near sea level to c. 780 m elevation (Mt Hauhungatahi, Wellington L.D.) on North I. and to at least 460 m (Fyffe-Palmer Scenic Reserve, Marlborough L.D. and near Bruce Bay, Westland L.D.) on South I. Commonly associated species include Hymenodon pilifer, Leptotheca gaudichaudii, Rhizogonium novae-hollandiae, R. pennatum, and Zoopsis argentea. Beever (1984) made a detailed study of tree-fern epiphytes in the Waitakere Ranges (N Auckland L.D.) and noted the occurrence of C. complanatum on 93% of sampled Cyathea dealbata, 100% of Dicksonia squarrosa, but only 2% of Cyathea medullaris.
The status of C. brownseyi Vitt & H.A.Mill. has been problematic in N.Z. from its description in 1995. Careful reading of the paper in which this taxon was described (Vitt 1995) suggests that the characters considered most significant by the describing authors are the nature of the upper margins of the lateral vegetative leaves and of the inner perichaetial leaves. These two characters seem unlikely to vary independently.
The presence of leaf margin denticulations indeed appears to be a feature restricted to material from the western coast of the South and Stewart Is. However, individual collections from that region are highly variable with respect to vegetative and perichaetial toothing, with variation occurring both within a single plant and between plants. The differentiation of material with and without marginal teeth seems to have been made arbitrarily in naming material in N.Z. herbaria. Both C. complanatum and C. brownseyi were described by Vitt (1995, in his key to species) as having a percurrent costa in the vegetative leaves. The variation of the vegetative leaf costa in N.Z. material is more variable than allowed by him and ranges from predominantly subpercurrent to short- or long-excurrent, often in the same plant.
Other features cited by Vitt in distinguishing C. brownseyi were the shape of the inner perichaetial leaves, their apical shape, and the thickness of their upper margins (unistratose vs partially bistratose). Perichaetial leaf characters in Calomnion are often particularly difficult to observe since many collections have few or no perichaetia. When present and intact, the shape of the inner perichaetial leaves is extremely variable and ranges from narrowly panduriform to narrowly spathulate to what Vitt usefully described as oblanceolate. There is no discernible pattern in this variation and it is here interpreted to be either developmental or environmentally induced and of little taxonomic value.
Vitt also cited the presence of partially bistratose perichaetial leaf margins as a character differentiating between the N.Z. "C. brownseyi" (unistratose) and C. melanesicum H.A.Mill. (partially bistratose). Some collections of N.Z. "C. brownseyi" develop small marginal bistratose patches, however, particularly at the base of marginal teeth.
After a study of a range of specimens in the herbarium (including the holotype and some paratypes of C. brownseyi) and in the field, N.Z. material of Calomnion is viewed here as a single variable species for which the oldest name is C. complanatum. Calomnion brownseyi is not recognised here. Material of C. melanesicum, the species most closely allied to C. brownseyi according to Vitt’s (1995) trees, has not been available for comparison.