- ≡ Dicranum bicolor Müll.Hal., Syn. Musc. Frond. 1, 392 (1848)
- = Campylopus bicolor var. intermedia R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 472 (1897)
- = Campylopus stewartii R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 472 (1897)
- = Campylopus ericeticola Müll.Hal. in Müller & Brotherus, Abh. Naturwiss. Vereins Bremen 16: 496 (1900) – as ericeticolus
- ≡ Campylopus bicolor var. ericeticola (Müll.Hal.) Dixon, Bull. New Zealand Inst. 3: 87 (1923) – as ericeticolus
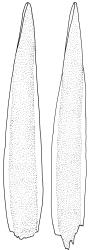
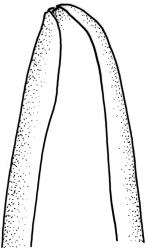
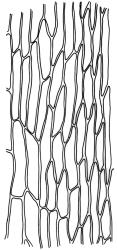
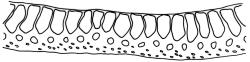
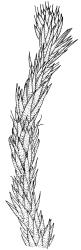
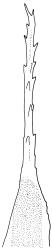
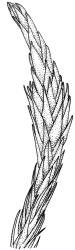
Plants yellow- to brown-green, mostly dark brown to nearly black below, forming dense tufts in wet habitats. Stems (5–)20–55 mm, not branched, in cross-section with a weakly defined central strand and thick-walled cortical cells; rhizoids absent or sparse on lower stem, brown. Leaves erect or erect-spreading, stiff, not altered when dry, not or only weakly comose, mostly linear-lanceolate, scarcely narrowed at base, subtubulose, variable at apex (mostly rounded, cucullate, and lacking a hair-point, sometimes acute and concolorous, and sometimes with a hyaline hair-point), entire or denticulate at apex (cucullate forms are entire except for projecting cell ends at extreme apex), with a very weak border that is scarcely visible above mid leaf, 3.0–6.5 × c. 1 mm (flattened); basal laminal cells thin-walled, not porose, hyaline, oblong or rectangular, mostly c. 30 µm, with an abrupt upper limit; mid and upper laminal cells incrassate, vermicular or linear-rhombic, variably porose, mostly c. 30–60 × 4–5 µm, mostly obliquely oriented; alar cells not forming a well-defined group; marginal cells narrower, forming a very weak and poorly defined border that ends far below apex. Costa c. 475–500 µm and mostly ≥80% of the leaf base width, filling the upper leaf (for c. ¼ the leaf length), percurrent or excurrent, lacking lateral spurs, in abaxial surface view cells linear and incrassate, in adaxial surface view cells linear-oblong and thin-walled, in cross-section (mid leaf) with c. 4 cell layers, with a single layer of median guide cells, a single adaxial layer of large and thin-walled hyalocysts, and 1–2 layers of abaxial stereids, lacking abaxial ridges. Propagula absent.
Dioicous. Perichaetial leaves strongly sheathing, narrowly acute from an oblong base, to c. 5 mm. Setae strongly cygneous, c. 8 mm; capsules ovoid, non-strumose, sulcate when dry, c. 1.3 mm; stomata absent, annulus strongly differentiated and revoluble; operculum rostrate from a conic base. Peristome teeth orange and vertically striolate below, hyaline above, split c. half-way to base. Calyptra fringed at base. Spores c. 18–21 µm, smooth.
Dixon 1923, pl. 7, fig. 9; Catcheside 1980, fig. 41; Bartlett & Frahm 1983, fig. 2 a–g; Frahm 1987, fig. 3; Meagher & Fuhrer 2003, p.139 (as C. bicolor var. bicolor).
This species is most likely to be confused with C. kirkii, q.v.
NI: N Auckland, including offshore islands (TK, GB), South Auckland (Rotorua, near Ātiamuri), Gisborne (Lake Waikareiti), Wellington (Mt Ruapehu); SI: Nelson (Pūponga, Bainham, Cobb Valley, Scarlett Range, Denniston Plateau, Paparoa Range, Victoria Range, Matiri Plateau), Canterbury (Lewis Pass, Alford Range, Mt Sebastopol), Otago, Southland (Ōtāpiri, Mavora Lakes, Te Ānau, Percy Saddle, Lake Manapōuri); St; Ch.
Australasian. Tasmania*, mainland Australia* including W.A. and N.S.W.; Scott & Stone (1976) reported it from other Australian states.
On peat, less commonly directly on rock, usually in acidic areas of impeded drainage (pākihi, bogs, over rock pavements, etc.) or seasonal waterlogging; sometimes forming extensive turves. Ranging in elevation from 10 m (Karikari Peninsula, N Auckland L.D.) to at least 1230 m (Scarlett Range, Nelson L.D.). A large fraction of the collections are from N Auckland L.D. Campylopus introflexus is frequently associated with C. bicolor. Catcheside (1980, p. 113) considered C. bicolor to occur "chiefly on wet rocks in gorges" in South Australia, while Meagher & Fuhrer (2003) considered it to be "often the dominant species on damp, flat granite outcrops, forming large mats of stiffly upright shoots" in southern Australia.
Well-developed forms of C. bicolor are distinctive and readily recognised, by virtue of the stiff, non-comose leaves, which have rounded and cucullate apices, vermicular or linear-rhombic and porose mid laminal cells, hyaline basal laminal cells, and inconspicuous alar cells; the costa occupies c. 80% of the leaf base, nearly fills the upper leaf, lacks spurs, and at mid leaf has a single adaxial layer of large, thin-walled hyalocysts. In abaxial surface view (using the compound microscope) the cells of the mid leaf costa lack ridges and have uniformly elongate cells, while in adaxial view the hyalocysts can be viewed.
However, this is a highly variable species, which varies greatly in leaf form, particularly apical shape and the degree of hair-point development. Campylopus bicolor is widely distributed in N.Z. but sporophytes are extremely rare. Capsules have been observed only in a W. Martin collection from Fraser Peak Flats, Stewart I. (CHR 449100).
The var. ericeticola (Müll.Hal.) Dixon is not recognised here. A significant fraction of populations of C. bicolor show transition from concolorous, ± cucullate leaves, to concolorous and acute leaves, to hyaline-tipped and piliferous leaves. The frequency of these intermediates and the many populations showing the full range of variability indicate that the var. ericeticola is untenable. Bartlett & Frahm (1983, p. 369) refer to the var. ericeticola as "at best a weak taxon." Frahm (1994, p. 315) discussed the variability of C. bicolor but drew no firm conclusions regarding the variety’s status. He stated initially that specimens with "both cucullate leaves and leaves with hair-points from the same plants" led "to the conclusion that the different leaf apices are the result of modification", and then stated that culture experiments (and a mixed N.S.W. collection) caused him to believe that the two alleged varieties represented different phenotypes. He claimed that the perigonial leaves were hair-pointed in both var. ericeticola and what he would have termed var. bicolor. Given the large fraction of herbarium collections showing transition from concolorous leaves to piliferous ones, his arguments in support of var. ericeticola are neither conclusive nor compelling.
Collections in which cucullate and piliferous leaves occur on the same shoots include material from Lake Waikareiti, Lewis Pass, and the Port Pegasus area of Stewart I.
Distinguishing between C. bicolor and C. introflexus can be exceedingly difficult, particularly when both piliferous and hyaline-tipped leaves are present; much herbarium material is incorrectly named. Populations of C. bicolor and C. introflexus can also grow inter-mixed. The two cannot always be differentiated in the field, although populations with abundantly piliferous and strongly reflexed leaf apices can assuredly be recognised as C. introflexus. The converse does not always hold true. Critical material can only be differentiated using a compound microscope.
The most useful distinctions are provided by the abaxial surface view (at ×320, using the fine focus) of the mid leaf costa. In C. bicolor the abaxial cells are uniformly linear and the costa surface lacks ridges, while in C. introflexus the cells of the ridges appear as ranks of short-rectangular or quadrate cells alternating with more elongate cells of the separating grooves. Secondly, the mid and upper laminal cells are vermicular to linear-rhombic and ± porose in C. bicolor, while in C. introflexus the mid and upper laminal cells are (at least partially) rounded-quadrate to rounded-oblate and lack pores. In both these species the alar cells are not or weakly differentiated. The hyaline basal laminal cells in C. bicolor are mostly shorter (c. 30 µm) and collectively have a less abrupt and less V-shaped transition to the mid laminal cells, while in C. introflexus the hyaline basal laminal cells are narrowly rectangular, commonly c. 45–105 µm, with a more abrupt V-shaped transition.
A notable growth form occurs on Mt Maungatua (Otago L.D.) in which the stems are elongate (to c. 50 mm) and the leaves 4–5 mm, evenly tapered from an oblong base to an acute or slightly rounded and subcucullate apex. In some Mt Maungatua material the formation of cucullate leaves near the stem apex and in bands along the stems facilitates recognition, but in many collections no cucullate leaves are present. In much of the Maungatua material the mid leaf laminal cells are shorter (c. 25–40 µm) than representative material for C. bicolor.
I agree with Bartlett & Frahm (1983, p. 369) that the tendency here to produce hair-points appears to be a response to periodic or seasonal desiccation, with broader and ± cucullate leaves produced during wetter periods or in wetter habitats. There is a tendency for hyaline-tipped leaves to have narrower-than-representative costae and less distinctly vermicular upper laminal cells. Frahm’s (1994) assertion that ♂ leaves are invariably hair-pointed cannot be confirmed.
Frahm’s (1987, 1994) discussions of the alleged variety ericeticola are mutually inconsistent. Additionally, a proposed subspecies, C. bicolor subsp. atroluteus (Müll.Hal.) J.-P.Frahm (combination made in Frahm 1985) from South Africa was subsequently rejected by Frahm (1994). Because of the uncertainty surrounding both the variety ericeticola and the African subspecies, neither the autonym C. bicolor subsp. bicolor nor C. bicolor var. bicolor are employed in this treatment.