- = Campylopus torquatus Mitt. in Wilson, Bot. Antarct. Voy. III. (Fl. Tasman.) Part II, 173 (1859)
- = Campylopus ohingaitii R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 470 (1897)
- = Campylopus sparksii R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 467 (1897)
- = Campylopus lonchochaete Müll.Hal. in Müller & Brotherus, Abh. Naturwiss. Vereins Bremen 16: 495 (1900)
Campylopus holomitrium sensu Sainsbury 1955, non C. holomitrium (Müll.Hal.) A.Jaeger. See notes below.
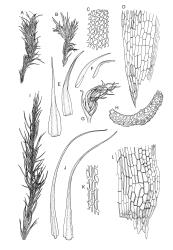
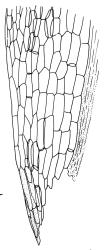
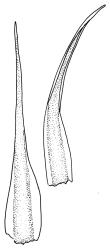
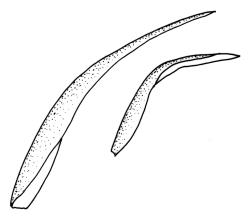
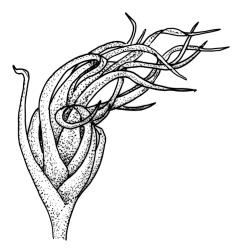
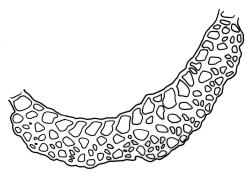
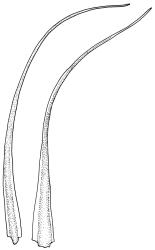
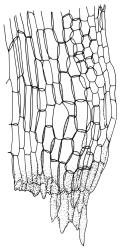
Plants yellow- or pale-green above, usually red-brown below, forming dense turves, often with masses of deciduous, pale, strongly falcate leaves above. Stems usually branched by innovation, (5–)10–40 mm, in cross-section with a large central strand, several layers of thick-walled subcortical cells and an outermost layer of thin-walled cells, densely matted with brick-red smooth rhizoids. Leaves usually erect-spreading or loosely secund when moist, little altered or contorted (especially near the shoot tips) when dry (see supplementary notes below), not or weakly comose, lanceolate from an oblong and pale base, rather abruptly narrowed to a long subula, strongly concave to subtubulose, lacking a hair-point but may be weakly hyaline at apex, entire below, mostly serrulate near apex, not bordered, mostly 3–6(–7.5) × c. 0.5–0.7 mm; basal laminal cells thin- to moderately firm-walled, hyaline, oblong or rectangular, not abruptly differentiated from the upper laminal cells, mostly 25–60 µm long; upper laminal cells mostly quadrate or short-rectangular but some irregular, neither oblate nor obliquely oriented, not porose, mostly 9–15(–30) × (6–)7–9 µm; alar cells not or weakly differentiated; marginal cells not differentiated. Costa c. 290–350 µm wide and c. 60–70% of the leaf base width, c. 140–180 µm wide and well defined at mid leaf (but mostly obscured by concavity of the leaf), filling the upper subula and excurrent, lacking lateral spurs; in abaxial surface view the cells rectangular, firm-walled, and c. 5 µm wide; in adaxial surface view the cells long-rectangular and thin-walled, c. 60–120 × 12–15 µm; in cross-section (mid leaf) c. 5–7 cell layers thick, with a median layer of guide cells, a single layer of exposed adaxial hyalocysts, and 2–3 layers of abaxial stereids, lacking abaxial ridges. Propagulae often present, consisting of deciduous, reduced, pale, and strongly falcate leaves scattered among the upper vegetative leaves.
Dioicous. Perichaetial leaves loosely sheathing, longer and more narrowly subulate than vegetative leaves. Perigonia aggregated in a terminal coma, surrounded by broadly ovate, concave, and ± pigmented bracts. Setae strongly cygneous, c. 7–9 mm; capsules erect and symmetric, ovoid, non-strumose, sulcate when dry, c. 1.0–1.5 mm; stomata absent; annulus well differentiated, revoluble; operculum rostrate from a conic base. Peristome teeth orange and vertically striolate below, hyaline above, split c. half-way to base. Calyptra strongly fringed at base. Spores 12–14 µm, nearly smooth.
Campylopus pallidus frequently grows with C. introflexus and can occasionally be difficult to separate from forms of the latter that lack a hair-point. The ± quadrate upper laminal cells and the smooth abaxial surface of the costa (at mid leaf) contrast with the more rhombic and often oblate cells and the abaxial costal ridges of C. introflexus. The mid leaf adaxial costal hyalocysts in C. pallidus are less regular in size and shape than those of C. introflexus. Campylopus pallidus is occasionally confused with Campylopodium lineare, but that species has narrower costae with adaxial stereids, longer upper laminal cells, larger spores, and a non-fringed calyptra.
NI: K; N Auckland, including offshore islands (TK, PK, LB, GB, RT), S Auckland, Gisborne (Lake Waikaremoana, Huiarua Range), Hawke’s Bay (Wairoa), Taranaki (Mt Messenger, Stratford Track on Mt Taranaki, Hawera), Wellington; SI: Nelson, Marlborough (Queen Charlotte Sound, Kenepuru, Kaikōura, Mt Fyffe), Canterbury (Arthur’s Pass, Banks Peninsula), Westland, Otago, Southland; St; Sn; A; Ant; C.
Austral. Tasmania*, mainland Australia*, Antarctica*. Reported from New Caledonia, southern South America, south-east North America, Europe, and China by Frahm (1994, as C. pyriformis).
Most often on stumps, rotten wood, and humic soil (including peat) in a wide range of forest or open habitats; rarely on trunks and branches of recently fallen trees (and apparently sometimes epiphytic). Much less commonly on mineral soils. Also occurring as a robust thermophilic growth form (sometimes termed Campylopus holomitrium sensu Sainsbury) in association with geothermal fumaroles in the central North I. The species is widespread throughout N.Z. but there are relatively few records from eastern regions of both main islands. The large number of collections from both peat and epiphytic habitats in Olearia lyallii scrub on Snares I. suggests that this is a widespread species on that island. Vitt (1979, as C. pallidus) indicated it to be present "only near areas of disturbance and colonization by man" and "disturbed peat surfaces" on Auckland Is. On the North I. ranging to at least 1300 m (Stratford Track, Taranaki L.D.) and on the South I. from near sea level (Awarua Bog, Southland L.D.) to at least 910 m (Old Man Range, Otago L.D.).
No material of C. pallidus has been confirmed from Macquarie I, although Seppelt (2004) recorded C. pyriformis from numerous sites on that island. His description, habitat notes, and fig. 52 suggest that the Macquarie material he illustrated is referable here. His fig. 52, 5, shows short-rectangular and non-obliquely oriented mid laminal cells, albeit with perplexing elongate marginal cells. Seppelt’s other illustrations, including his costal cross-sections, and his habitat notes are likewise suggestive of C. pallidus, a species that is documented from Auckland and Campbell Is.
This species is morphologically variable and occupies a range of substrates. The rather finely subulate, homomallous or slightly secund leaves, the abundant brick-red rhizoids, and the pale overall coloration give most collections of C. pallidus a characteristic habit. The colour of the rhizoids often fades in herbarium specimens. Microscopic examination (including a cross-section of the mid leaf costa) to confirm the quadrate or short-rectangular upper laminal cells, the absence of a well-defined alar group, and the nature of the costal cross-section are often required for confident identification. Non-representative growth forms are discussed below.
Propagule-like reduced shoots (mostly ≤5 mm long) with contorted leaves can be present near the apex of the main shoots. Each of these propagule-like plantlets can bear numerous pale, strongly curved ("boomerang"), and deciduous leaves. Both the propagule-like shoots and deciduous leaves appear to be most abundant on plants on rotten wood and subject to water stress. In some populations these shoots are numerous and grow directly on rotten wood; they may constitute most or all of the population. The leaves of the reduced shoots are smaller (usually <4 mm long) than leaves of more representative material and there is no clear size distinction between them and the falcate deciduous leaves. When present, deciduous leaves provide a reliable field character for the species; the deciduous leaves have firm-walled cells to their base and costae less well developed than in vegetative leaves. Reduced-stature (and propagule-like) plants almost certainly function as propagulae, as do fragmenting plantlets in other species of Campylopus.
The evidence for applying the European name C. pyriformis (Schultz) Brid. [Bryol. Univ. 1: 471 (1826)] to N.Z. material is not convincing. The application of this name by several authors in Australasia dates from a brief publication by Corley & Frahm (1982). That publication outlined some confusion around the type of the basionym (Dicranum pyriforme Schultz) and confusingly stated that material described from the Azores as C. azoricus Mitt. "must be regarded as the typical form of C. pyriformis". Corley & Frahm placed the Australasian C. pallidus Hook.f. & Wilson in the synonymy of C. pyriformis. The situation is rendered even more perplexing by their subsequent statement that the species in question is an Austral or Australasian species that may have been introduced to Europe in "pre-botanical" times.
Subsequent publications, many authored by Frahm, have repeatedly cited the conclusions presented by Corley & Frahm (1982). Firmer evidence that Australasian material is conspecific with a European (Azorean?) species is required before the name C. pyriformis can be confidently applied to the N.Z. material. It is preferable to utilise the earliest Australasian name that can be typified for this widespread (in N.Z., Tasmania, and mainland Australia) and variable species. The name C. pallidus Hook.f. & Wilson is based on ample fruiting material from N.Z., and this name is applied here. The application of this name agrees with that favoured by Scott & Stone (1976), but is at odds with the use of C. torquatus Mitt. in Wilson by both Dixon (1923) and Sainsbury (1955).
The taxonomic status and the correct name for what has been named C. holomitrium auct. has long been a source of confusion in N.Z. The name C. holomitrium auct. [including sensu Sainsbury 1955 but specifically excluding C. holomitrium (Müll.Hal.) A.Jaeger, which is Campylopodium capillaceum] has been applied by N.Z. workers to a growth form of Campylopus pallidus associated with geothermal fumaroles in the central North I. This thermophilic growth form is usually darker or more yellow-green than representative material of C. pallidus. In the thermophilic growth form the stems are commonly longer, 40–60(–120) mm. Vegetative leaves are longer (c. 6–7.5 mm), the area of hyaline basal laminal cells is reduced, there is a tendency for the alar cells to become enlarged and pigmented, and the mid laminal cells are somewhat more elongate (to c. 30 × 6 μm) The larger stature seems predictable in populations growing in a constantly very warm and hypermoist environment. A few propagulae can occasionally be found on the upper stems of herbarium specimens of the thermophilic growth form. The propagulae often occur in very compact terminal clusters. J. Beever (in litt., 9 Jan. 1998) reported that curved brood leaves could be found at Karapiti (e.g., CHR 413398, Wellington L.D.) on "individual stems which are taller than those surrounding them, or when chunks of the moss have been dislodged from the sward some months previously and are lying loose." J. Beever suggested (in litt., 9 Jan. 1998) that C. holomitrium sensu Sainsbury is merely a robust form of what she termed C. pyriformis. Her interpretation is accepted here, albeit with the appropriate name difference.
The ecology of the vegetation at Te Kopia geothermal field (Wellington L.D.), where the thermophilic growth form of C. pallidus is a dominant component of the geothermal vegetation, has been discussed by Burns (1997). At this site C. pallidus (cited as C. capillaceus) was the most abundant groundcover on sites with soil temperature of >50 deg. C at a depth of 15 cm. Low soil pH and high concentrations of aluminium were also significant edaphic factors in explaining vegetation patterns at Te Kopia.
Type material of Dicranum distractum Müll.Hal. has not been seen. It was collected by either H. Schauinsland or W. Wacker in N.Z., with no further locality data in the protologue. It was included in the synonymy of C. holomitrium (Müll.Hal.) A.Jaeger by Dixon (1923) and likely belongs in the synonymy of C. pallidus.
The name Dicranum holomitrium Müll.Hal. [Syn. Musc. Frond. 1: 389 (1848)] and its homonym Campylopus holomitrium (Müll.Hal.) A.Jaeger are synonyms of Campylopodium capillaceum (Hook.f. & Wilson) Fife, comb. nov. (in this publication). The name Campylopus capillaceus Hook.f. & Wilson [London J. Bot. 3: 543 (1844)] is an earlier name for what has often been termed Campylopodium medium (Duby) J.-P.Frahm & Giese.