- ≡ Dicranum cardotii R.Br.bis, Trans. & Proc. New Zealand Inst. 35: 329 (1903)
- ≡ Anisothecium cardotii (R.Br.bis) Ochyra, Moss Fl. King George Island Antarctica 114 (1998)
- = Dicranella wairarapensis Dixon, Bull. New Zealand Inst. 3: 65 (1914)
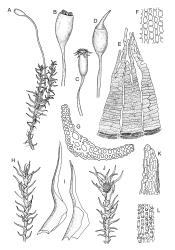
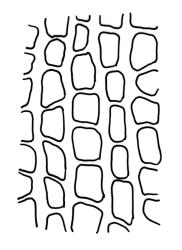
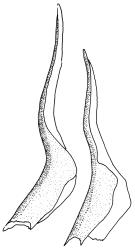
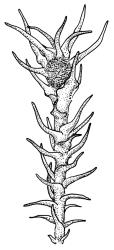
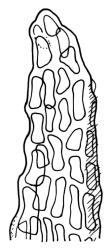
Plants medium to large, bright yellow-green, forming dense turves. Stems from c. 10 to at least 70 mm, in cross-section with a distinct central strand and 2–3 layers of firm-walled cortical cells. Leaves abruptly wide-spreading from an erect and sheathing base, contorted when dry, abruptly tapered from an oblong base to a narrowly triangular, spreading, and bistratose limb, with a very strongly defined shoulder, broadly acute or rounded at apex, crenulate at margins due to bulging cells, (1.1–)1.3–2.6(–3.5) × 0.6–0.7 mm, broadly U-shaped in cross-section, plane at margins; cells of mid limb short-rectangular, c. 4–6 µm wide and 1.5–3:1, firm-walled, mostly bistratose, bulging-mammillose (mammillae mostly 2 per cell and located at cells ends); cells of leaf base oblong-rectangular, variable in length (commonly c. 40–75 × 5–7 µm), smooth. Costa occupying c. ⅕ of the leaf base, well-defined, subpercurrent, not filling the limb, in cross-section with median guide cells and small abaxial and adaxial stereids groups, the adaxially exposed cells mammillate and more elongate than adjacent laminal cells. Tubers occasionally (mostly in depauperate populations) present on rhizoids, brown, spherical, 75–180 µm diam.
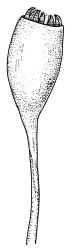
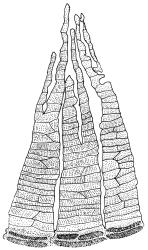
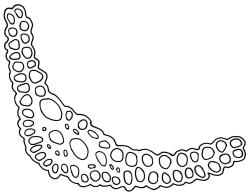
Dioicous. Perichaetia often several per stem, with leaves enlarged. Setae (6–)10–20 mm, flexuose and stout, sinistrorse, red-brown; capsules erect, short-cylindric, 1.0–1.3 mm, not strumose, constricted below the mouth and very weakly furrowed when dry (due to thickened longitudinal cell walls); mouth transverse; exothecial cells ± rectangular or quadrate, arranged in distinct columns, with incrassate longitudinal walls and relatively thin transverse walls; stomata not seen; annulus apparently absent; operculum obliquely rostrate, (0.7–)1.0–1.5 mm. Peristome teeth inserted c. 90 µm below rim, c. 300 × 75 µm, connate in basal 75–90 µm, each tooth divided ½ to ⅔ to the base into 2 uneven forks, which are often irregular in outline and ± anastomosed (adjacent teeth also occasionally anastomosed), often irregularly perforate, the outer surface of the lower tooth bearing fine trabeculae traversing the full width of the tooth, the inner surface with a zig-zag line that divides the tooth vertically into asymmetric segments, the lower tooth in surface view appearing irregularly and very finely papillose (not papillose-striolate) below. Calyptra as per genus. Spores (21–) 24–36 µm, green, smooth.
Dixon 1914, pl. 6, fig. 15 (as D. wairarapensis).
In a N.Z. context, D. cardotii is most likely to be confused with D. vaginata. Dicranella cardotii has a narrow costa, which does not entirely fill the limb and crenulate leaf apices (the projections of the mammillate cells are not visible under the stereoscope), in contrast to a stouter costa that wholly or nearly fills the limb and few to many large and irregular teeth near the leaf apex (visible under the stereoscope) in D. vaginata. The peristome teeth in D. cardotii are split ½ to ⅔ their length, while those of D. vaginata are split nearly to their base. Mature capsules of D. cardotii appear weakly furrowed when dry (due to the exothecial cells being arranged in vertical ranks and having thickened longitudinal walls), while those of D. vaginata are not furrowed when dry and lack differentially thickened cell walls. Dicranella cardotii is more widespread and extends to higher elevations regionally than does D. vaginata.
Dicranella cardotii has leaves more strongly sheathing and spreading when dry than does the superficially similar Chrysoblastella chilensis. The leaves of C. chilensis, by contrast, are more erect-appressed when dry and firmer in texture; in cross-section the bistratose lamina and the strongly bulging-mammillose laminal cells of C. chilensis are unmistakeable. Dicranella cardotii is a plant of wet, often shaded sites whereas C. chilensis occurs in drier and more insolated habitats.
NI: S Auckland (Mt Maungapōhatu, Wairākei Valley), Hawke’s Bay (Waikari Gorge, Tarawera), Taranaki, Wellington; SI: Nelson, Marlborough (Isolated Creek, Waimā River), Canterbury, Westland (Blacks Point), Otago, Southland; St; A; C; M.
Australasian or austral. Tasmania*. Reported from mainland Australia by Scott & Stone (1976, p. 148). This species almost certainly is widespread in southern South America, but there is considerable confusion surrounding several closely allied species, and their names. Until the confusion is eliminated it is expedient to consider D. cardotii as an Australasian species. Material that is morphologically highly similar to N.Z. material has been examined from Tierra del Fuego, Chile, and South Georgia.
Forming turves, often extensive (to at least 1 m × 1 m) on rocks, gravel, sand or humic soil at stream margins or in irrigated areas. Dicranella cardotii forms distinctive and often extensive yellow-green turves at stream margins and irrigated ledges in the eastern ranges of the South I. It is poorly documented in Westland and wetter parts of Southland and relatively few collections have been seen from the North I. Frequently associated species include Bryum laevigatum, Ochiobryum blandum, Philonotis pyriformis, and Tridontium tasmanicum. On the South I. ranging from near sea level (Fox River, Nelson L.D.) to c. 1650 m (Temple Basin, Canterbury L.D.).
The lower ½ or more of the peristome teeth in D. cardotii appear very finely papillose (with papillae <1 µm diam.) under the compound microscope. The upper ½ of the teeth appear ± striate. The papillae in the lower ½ of the teeth are irregularly arranged (e.g., as in J. Child 1563 from Herbert, Otago L.D., CHR 429080A), rather than regularly and vertically arranged (papillose-striolate) as they are in most Dicranella spp.
Rhizoidal tubers have been seen in few collections of D. cardotii (e.g., A.J. Fife 4679 from Four Mile River, Nelson L.D., CHR 103571). Tubers appear to be rarely produced in this species and have not been observed in well-developed populations. Plants in which the leaves exceed 2.6 mm are relatively rare and are usually associated with submerged habitats.
Dicranella cardotii R.Br.bis. has been listed by Ochyra et al. (2008) as a synonym of Dicranella campylophylla (Taylor) A.Jaeger [Basionym: Dicranum campylophyllum Tayl., London J. Bot. 7: 281, 1848]. Dicranella campylophylla (Taylor) A.Jaeger is based on an 1847 W. Jameson collection from Mt Pichincha in Ecuador, of which a portion is present in the Mitten herbarium (NY 267969). The application of the name D. campylophylla is much confused and clarity is required concerning its taxonomic and nomenclatural relationships with other South American taxa. These include, particularly, the species originally named as Aongstroemia hookeri Müll.Hal. [Syn. Musc. Frond. 2: 607, 1851], Anisothecium jamesonii Mitt. [J. Linn. Soc., Bot. 12: 39, 1869], and Aongstroemia persquarrosa Dusén [Ark. Bot. 4(1): 11, 1905; Syntype: Chile, Patagonia occ., valle fluminio Rio Aysén, in rupibus irrosatis, P. Dusén, Feb. 1897, NY 267998!]. All three of these names have combinations in Dicranella and, together with D. campylophylla, are certainly easily confused and could be synonymous with D. cardotii. However, the taxonomic and nomenclatural morass surrounding these South American names is beyond the ambit of this treatment, and requires a large-scale regional revision.
Some notes on the pertinent South American types may suggest pathways for future work.
Ochyra et al. (2008) provided a detailed description and illustration of his concept of Dicranella campylophylla and indicated that D. cardotii was synonymous. They distinguished Dicranella campylophylla and D. hookeri on the basis of laminal cell ornamentation, but this distinction is unconvincing. Hermite I. isotype material (NY 00012531!) of Aongstroemia hookeri Müll.Hal., collected by J.D. Hooker, has prorate laminal cells, conflicting with Ochyra’s statement that this species has laminal cells "smooth throughout". If the Hermite I. collection were from N. Z., I would not hesitate to name it as Dicranella cardotii R.Br.bis.
Anisothecium jamesonii Mitt. [J. Linn. Soc., Bot. 12: 39, 1869] is based on five syntypes from widely separated South American localities. Four of five syntypes of this name have been examined. They have strongly sheathing and shouldered vegetative leaves and short, nearly erect capsules, strongly suggestive of N.Z. D. cardotii and Ochyra’s concepts of both D. campylophylla and D. hookeri. The syntype from "loco Huambato in paludo, 9000 ped.", R. Spruce 34 (NY 12527!, CHR 461362!), if collected in Australasia, would unhesitatingly be referred to D. cardotii.
The misapplication of the name D. jamesonii (Mitt.) Broth. to the plant that is in this Flora termed D. schreberiana (Hedw.) H.A.Crum & L.E.Anderson appears to date from the discussion of Dixon (1914, p. 66).
The variability, taxonomy, and nomenclature of South American species of Dicranella with strongly sheathing and abruptly shouldered leaves and short, nearly erect capsules need to be clarified in a monographic context, but this is beyond the ambit of this Flora; once this is done, the name applied to this N.Z. species may change. Until that time it is preferable to apply an unambiguous and typifiable name to N.Z. material; Dicranella cardotii R.Br.bis is thus used in this Flora.