- ≡ Dicranum vaginatum Hook., Pl. Crypt. Pl. 3B (1816)
- ≡ Anisothecium vaginatum (Hook.) Mitt., J. Linn. Soc., Bot. 12: 39 (1869)
- = Dicranum clathratum Hook.f. & Wilson, London J. Bot. 3: 542 (1844)
- ≡ Dicranella clathrata (Hook.f. & Wilson) A.Jaeger, Ber. Thätigk. St. Gallischen Naturwiss. Ges. 1870–1871: 382 (1872)
- ≡ Anisothecium clathratum (Hook.f. & Wilson) Broth., Nat. Pflanzenfam., ed. 2 [Engler & Prantl] 10, 177 (1924)
- ≡ Dicranella vaginata var. clathrata (Hook.f. & Wilson) Sainsbury, Bull. Roy. Soc. New Zealand 5: 103 (1955)
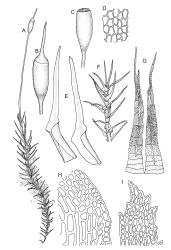
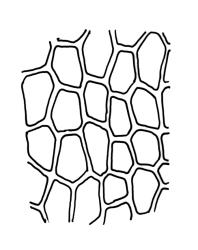
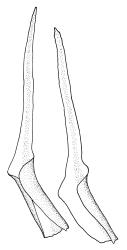
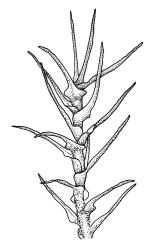
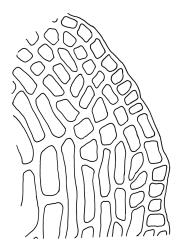
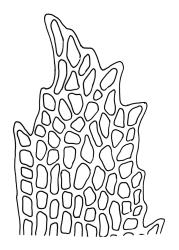
Plants medium-sized to large, yellow-green to dark brown to nearly black, forming dense turves. Stems orange or red, erect or creeping, branching by both innovation and forking, (7–)12–150 mm, in cross-section with a weak central strand and 2 layers of firm-walled cortical cells. Leaves wide-spreading from a strongly sheathing and pale base when moist, slightly more erect, spreading (or wide-spreading) and not contorted dry, abruptly tapered from the oblong and sheathing base to a rather broad and untwisted limb, with strongly defined shoulders, broadly rounded, obtuse, or broadly acute and with a few to many rather large, irregular teeth near apex, mostly (1.8–)2.0–2.6(–3.5) × c. 0.5–0.7 mm, weakly concave above, plane throughout or reflexed at shoulders; laminal cells at mid limb absent or obscure and short-rectangular, c. 3–5 µm wide and 1–2:1, firm-walled, smooth; cells of leaf base narrowly oblong-rectangular, variable in length (commonly c. 75–95 × 6–9 µm), smooth, becoming short-rectangular to quadrate at shoulder. Costa occupying c. ⅕ the width and ill-defined in leaf base, percurrent, stout and filling the limb (no lamina visible in cross-section of mid limb), in cross-section with median guide cells and small abaxial and adaxial stereid groups and with the cells on the adaxial surface more elongate than adjacent laminal cells. Tubers apparently absent.
Dioicous. Perichaetia with leaves to c. 4 mm. Perigonia terminal but often overtopped by innovations. Setae 9–14 mm, flexuose, weakly sinistrorse, red-brown; capsules erect, short cylindric, not constricted below the mouth when dry, 1.3–1.8 mm, neither strumose nor furrowed when dry, red-brown to nearly black and lustrous when dry; mouth transverse; exothecial cells oblong-hexagonal, firm-walled (longitudinal walls not differentially thickened); stomata superficial at capsule base; annulus poorly differentiated or absent; operculum long and finely rostrate from a conic base, 1.5–2 mm long. Peristome teeth c. 370 × 65 µm, divided nearly to base into two unequal forks (rarely with a third), occasionally narrowly perforate nearly to base, the outer surface of the lower tooth bearing fine trabeculae traversing the full width of the tooth, zig-zag line on inner surface not apparent, the lower tooth in surface view appearing nearly smooth or irregularly and very finely papillose (not papillose-striolate), the individual forks (upper ⅔ or more of the tooth) coarsely diagonally striate (apparently on both surfaces) and sometimes becoming baculate near apex. Spores 21–30 µm, green, smooth.
Although D. vaginata is sometimes confused with D. cardotii (which occupies similar habitats), in the majority of instances the two species can be distinguished in the field by the nature of the limb, which in the present species is stouter, stiffer, and completely or very nearly completely filled by the excurrent costa; other differences are detailed under D. cardotii.
Dicranella vaginata can also be confused with D. dietrichiae. The latter species is rarer, occupies mainly non-riparian habitats, and has a more restricted and northern distribution. It differs morphologically from D. vaginata by having shorter stems, much-less-pronounced shoulders, a leaf apex that is toothed by cells projecting on all surfaces of the percurrent costa, and sulcate capsules with a well-differentiated and persistent annulus.
Dicranella vaginata has been at times confused with Ditrichum strictum. Dicranella vaginata is a smaller and usually brighter-green plant, with leaves spreading to widely spreading and untwisted in both moist and dry conditions. By contrast, Ditrichum strictum is generally a larger and darker (dark or olive-green to black) plant with leaves ± erect and homomallous to weakly secund in both wet and dry conditions. Its leaves are mostly weakly twisted (spiralled) when dry. The leaves of Dicranella vaginata are shorter, mostly 2.0–2.6 mm long, while those of Ditrichum strictum are mostly 6–7 mm long. The narrowing at the shoulder is more abrupt, the sheathing nature of the leaf base more conspicuous in both fresh and dried conditions, and the lower costae ill-defined and narrow in Dicranella vaginata, while in Ditrichum strictum the narrowing of the leaf is more gradual, the sheathing nature of the leaf base less obvious, and the lower costae are clearly defined and broader (c. ⅓ the leaf base in width) in the lower leaf. Dicranella vaginata is dioicous while D. strictum is frequently (or always?) autoicous. When fruiting the spores are mostly smaller in D. vaginata (21–30 vs mostly 27–45 µm). The peristome is better developed here, with teeth c. 370 µm long and persistent vs c. 210–240 µm long and fugacious in Ditrichum strictum. The annulus in D. vaginata is poorly differentiated or absent, while in Ditrichum strictum the annulus is compound and revoluble. Dicranella vaginata occurs over a wide elevation range (from near sea level upwards), while Ditrichum strictum is not known below c. 950 m elevation on the main islands.
NI: N Auckland including offshore islands (GB, LB), S Auckland, Gisborne (Lake Waikaremoana, Wairoa), Taranaki (Otūmangahū Landing on Whanganui River, Mt Taranaki), Wellington (Mangapurua Landing on Whanganui River, Tongariro Forest Park, Mauriceville); SI: Nelson, Westland, Southland (Lake Manapōuri, Mosquito Creek near Riverton); St.
Austral-Andean. Ecuador* and Venezuela*. Cardot (1908, p. 60) recorded it from Colombia, while Crum (1994) recorded it from additional localities, mostly in Central America and the West Indies.
Stream banks, sometimes at the margins of cascades and often partially submerged. Occasionally in roadside ditches. Occurring on a variety of substrates, including sand (sometimes derived from granite or gneiss), granite, limestone, mudstone, and sandstone. At the middle reaches of the Whanganui River (forming the boundary between Wellington and Taranaki L.D.). D. vaginata forms turves of many square metres on papa (mudstone) and appears to be a dominant plant species on papa banks below the river flood level. On the North I. ranging from c. 15 m (Wairoa) to at least 275 m (Lake Rotoiti, S Auckland L.D) elevation; on the South I. ranging from 15 m (near Haast, Westland L.D) to c. 830 m (Herbert Range, Nelson L.D.) elevation. On the South I. this species is distributed exclusively in western regions. Ochiobryum blandum appears to be the most consistent associate; others include Blindia magellanica, irrigated forms of Distichophyllum pulchellum, Fissidens rigidulus, and Tridontium tasmanicum.
The pale sheathing leaf bases and stout, widely spreading limb, which is completely or nearly filled by the costa and coarsely toothed at its apex (with the teeth visible under the stereoscope), combined with the turf-forming habit and streamside habitat, characterise D. vaginata. The coarse diagonal striations conspicuous on the upper peristome teeth do not occur in any other N.Z. species of Dicranella. The diagonally striate-baculate nature of the peristome tooth forks are probably what Dixon (1914) had in mind when he stated "the filiform branches are densely and very highly cristate with close papillae, so that their internal structure is entirely hidden". This feature can be clearly observed in K.W. Allison 1798 from Waipoua Forest (CHR 532213).
Apart from type material of the tropical South American Dicranum vaginatum Hook. (1816), relatively little South American material has been examined. (The original protologue has been unavailable, and only the protologue in Hooker’s Musci Exot. 2: 141, 1820 has been seen.) One of the five South American specimens of Anisothecium vaginatum (Hook.) Mitt. cited by Mitten (1869, p. 39) is present in CHR: R. Spruce 36, collected at "Andes Quitensis, ad fl. Pastasa infra Banos, 5500 ped." (CHR 461364). Neither of these specimens seems distinguishable from N.Z. material in any meaningful way, including peristome structure.
The type material of Dicranum clathratum and the several combinations based on it bears no locality data beyond "New Zealand" and no reference to the "falls of the Kidi-Kidi River" (probably Kerikeri River, N Auckland L.D.) mentioned in the protologue. Nevertheless, there can be little doubt that the specimen cited above was used by Wilson in the preparation of the species description.
Both Dixon’s (1914, p. 65) and Sainsbury’s (1955, p. 103) comments concerning the nature of the peristome are confusing, and at least in Dixon’s case may have been influenced by his confusion of the present species (cited as D. clathrata) with D. campylophylla (cited as D. cardotii). Their observations do not accord with mine, and Sainsbury’s (1955, pl. 17, fig. 1) illustration is particularly misleading. The N.Z. material examined has peristome teeth divided nearly to the base into two unequal forks. Only in rare instances (e.g., A.J. Fife 6024b from Nile River, Nelson L.D., CHR 405664) do individual teeth occur with a third rudimentary projection. Perforations are relatively uncommon, occur in only a small fraction of the individual teeth in a single peristome, and are usually narrow and restricted to the basal c. 125 µm of the tooth. The N.Z. name Dicranum clathratum and its various combinations are unworthy of separation from the South American D. vaginata.
Dixon’s understanding of the relationship between the present taxon, Dicranum cardotii R.Br.bis, and Dicranum cockaynii R.Br.bis is confusing. Dixon (1914, p. 64; see also 1923, p. 77) cited six names, which he considered conspecific (or provisionally conspecific) with his concept of Dicranella clathrata. This synonymy is not quoted here.
Dicranella vaginata var. longifolia Dixon & Sainsbury in Sainsbury [Trans. R. Soc. N.Z. 75: 171, 1945] is synonymous with Ditrichum punctulatum Mitt., q.v.