- ≡ Gymnostomum laxum Hook.f. & Wilson, Bot. Antarct. Voy. I. (Fl. Antarct.) Part II, 399 (1847) – as Gymnostomum (Physcomitrium) laxum
- ≡ Physcomitrium laxum (Hook.f. & Wilson) Müll.Hal., Syn. Musc. Frond. 2, 546 (1851)
- ≡ Funaria laxa (Hook.f. & Wilson) Broth., Deutsche Sudpolar-Exped. 1901–1903, 8, 88 (1906)
- = Funaria subattenuata Broth., Öfvers. Finska Vetensk.-Soc. Förh. 40: 173 (1898) – as Funaria (Entosthodon) subattenuata
- = Meesia craigieburnensis R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 464 (1899)
- = Tayloria maidenii Broth., Proc. Linn. Soc. New South Wales 41: 583 (1916)
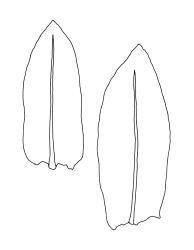
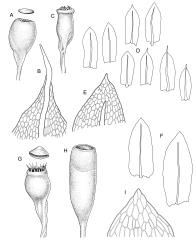
Plants yellow- or bright green, gregarious. Stems pale to red-brown, to 15 mm, branching once by subperigonial innovation, or forking beneath soil surface and producing subperigonial innovations above, beset with cerise rhizoids. Leaves erect-spreading, lingulate, 1.5–3.0(–4.0) × 0.6–1.0 mm, plane to weakly concave, entire or weakly crenulate above, tapered in upper ⅓, broadly acute or obtuse; upper laminal cells oblong-hexagonal, c. 70–100(–130) × 24–30(–45) µm, longer and more oblong below but not thinner-walled; marginal cells not differentiated or rarely weakly differentiated above; apical cell 25–55 µm (rarely to 100 µm); alar cells not differentiated or slightly more pigmented than adjacent cells. Costa rather thin and weak, c. 30–40(–50) µm wide near base, failing c. 5–10 cells (rarely more) below leaf apex. Axillary hairs present.
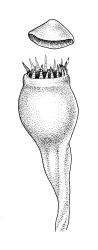
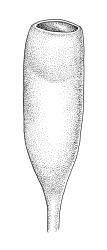
Autoicous. Perigonia and perichaetia as per genus. Setae (5–)10–20(–23) mm, dextrorse above, pale brown, weakly hygroscopic; capsules erect, symmetric, oblong-obovoid or oblong-pyriform, scarcely constricted below mouth when dry, 1.5–2.5(–3.0) mm, with a well-differentiated neck ⅓ to ½ the capsule length, red-brown at maturity; mouth c. ¾ the capsule diameter, transverse; exothecial cells with distinct lumina, c. 2–3:1, averaging 30–40 µm, in cross-section with anticlinal walls not or very weakly cuneate, c. 6–8 rows isodiametric to oblate and darker at capsule mouth; operculum mammillate or strongly convex. Peristome double; exostome teeth straight, cerise, often fugacious, to 300 × 50–75 µm but often shorter and irregular, acute or rounded, baculate-papillose, not or weakly striate, weakly nodulose (lacking marginal appendiculae), inner surface scarcely trabeculate; endostome rudimentary and fugacious, with segments irregular and hyaline, to 120 µm, gemmate to lowly insulate. Calyptra mitrate or splitting on one side to become cucullate, rostrate. Spores 25–35 µm, gemmate to lowly insulate.
Fife 1987a, figs 1–21; Fife 1987b, figs 91–116; Malcolm & Malcolm 2003, p. 26.
NI: Gisborne (near Lake Waikareiti), Wellington (Mt Ruapehu, Mt Ngāuruhoe, Northwest Ruahine Range); SI: Nelson, Canterbury, Westland, Otago, Southland (Eyre Range); A; M. No indisputable collections from St or C have been confirmed, but it almost certainly occurs on those islands.
Austral-Andean. Tasmania*, mainland Australia (Australian Alps)*, Kerguelen*, Marion I.*, Crozet Is*, Chile*, Bolivia*, Peru*, Ecuador*, Venezuela*.
On waterlogged to sometimes mesic and usually shaded humic soil (usually with sand or gravel fragments). Often at the margins of small subalpine or alpine streams; also on wet outcrops or in seepages. Occurring in areas with various bedrock types, including greywacke, schist, granite, limestone, and ultra-mafics. It is frequently shaded by overhanging snow tussocks. From c. 900 (Tongariro National Park, Wellington L.D.) to c. 1900 m (Makatote River headwaters, Mt Ruapehu, Wellington L.D.) on the North I. and from c. 730 (Broken River Basin, Canterbury L.D.) to at least 1650 m (Mt Arthur, Nelson L.D.) on the South I. On Auckland I. it grows to near sea level. Frequently associated flowering plants include Nertera depressa, Pratia angulata, and Drosera spp. (especially D. arcturi), while associated bryophytes can include Breutelia pendula, Blindia robusta, aquatic growth forms of Distichophyllum pulchellum, Isotachis montana, Jamesoniella tasmanica, and members of the Aneuraceae.
The broadly acute or obtuse, lingulate leaves, the ± mammillate opercula, and oblong-obovoid capsules, and the bright cerise (cherry-coloured) rhizoids and exostome teeth facilitate recognition of this species, as does its predominantly subalpine to alpine distribution. Microscopically, the short exothecial cells with distinct lumina and non-cuneate anticlinal cell walls are very distinctive.
The only other regional Entosthodon with which E. laxus could be confused is E. subnudus var. gracilis, which is a much smaller lowland plant with plano-convex opercula. A review of the ecology, world distribution, and extensive synonymy of E. laxus was provided by Fife (1987a).