- ≡ Mertensia dicarpa (R.Br.) Poir., Encycl. Suppl. 3, 670 (1814)
- ≡ Platyzoma dicarpum (R.Br.) Desv., Mém. Soc. Linn. Paris 6: 199 (1827)
- ≡ Calymella dicarpa (R.Br.) C.Presl, Gefässbündel Farrn 30 (1847)
- ≡ Pteris dicarpa (R.Br.) Christenh. in Christenhusz et al., Phytotaxa 19: 22 (2011)
- = Gleichenia hecistophylla A.Cunn., Companion Bot. Mag. 2: 361 (1837)
- ≡ Gleichenia semivestita var. hecistophylla (A.Cunn.) Hook.f., Bot. Antarct. Voy. II (Fl. Nov.-Zel.) Part II, 5 (1854)
- ≡ Gleichenia circinnata var. hecistophylla (A.Cunn.) Hook.f., Handb. New Zealand Fl. 348 (1864)
- ≡ Gleichenia dicarpa var. hecistophylla (A.Cunn.) G.Schneid., Book Choice Ferns 2, 219 (1893)
- ≡ Gleicheniastrum hecistophyllum (A.Cunn.) Nakai, Bull. Natl. Sci. Mus. 29: 44 (1950)
- = Gleichenia dicarpa var. major T.Moore, Index Fil. 375 (1862)
- ≡ Gleicheniastrum hecistophyllum var. majus (T.Moore) Nakai, Bull. Natl. Sci. Mus. 29: 44 (1950)
- = Gleichenia dicarpa var. longipinnata T.Moore ex G.Schneid., Book Choice Ferns 2, 220 (1893)
- = Calymella major Nakai, Bull. Natl. Sci. Mus. 29: 41 (1950)
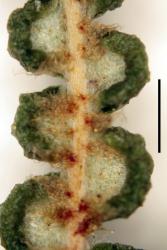
Rhizomes long creeping, 1–3.5 mm diameter; rhizome scales lanceate or ovate, 0.7–2.7 mm long, 0.2–1.1 mm wide, brown, setose and/or ciliate, usually also with smaller stellate scales. Fronds 80–1460+ mm long. Stipes 50–1100 mm long, usually distally scaly, rarely glabrous. Laminae 30–730+ mm long, 30–540+ mm wide. Rachis buds not extending or extending 1–3 (rarely 4) times; rachis bud scales lanceate or ovate, 0.7–4.0 mm long, 0.3–1.0 mm wide, brown, ciliate, sometimes apically setose. Rachis buds usually with single or paired accessory leaflets near base of each proximal-most costa, or rarely without accessory leaflets; accessory leaflets where present usually proximally banched. Pinnae 35–500+ mm long, 15–420 mm wide; with 1–4 (rarely 0 or 5) successive pseudodichotomous forks (excluding growth from pinna buds); pinna buds occasionally extending once (rarely 2–3 times). Proximal-most costae 11–120 mm long, persistently scaly but glabrescent in old fronds. γ costae (where not proximal-most costa) 10–105 mm long, with 2–15 (rarely 0) pairs of costal leaflets (excluding accessory leaflets around bud). β costae 29–250 mm long, with 7–50 pairs of ultimate leaflets; adaxially usually with brown or pale branched scales with curly branches that form a lanate mass, or rarely with dark-brown or pale stellate scales with ± patent branches; abaxially with a mix of ovate or lanceate, brown or pale, ciliate and/or setose scales, 420–1400 µm long, 150–450 µm wide, and dark brown, red-brown or pale stellate scales with ± patent or curled branches, 150–500 µm long. Longest ultimate leaflets 13–90 mm long, with 17–80 pairs of ultimate leaflets. α costae adaxially with pale, branched, hair-like scales, either glabrescent and not percurrent, or persistent and percurrent; abaxially with lanceate to ovate, brown or pale (and then usually with darker attachment) ciliate scales, 400–900 µm long, 150–520 µm wide, often restricted to proximal half of α costae, or occasionally percurrent. Ultimate segments 0.4–1.6 mm long, 0.6–1.6 mm wide, ± square, abaxially pouched or recurved, apices rounded; adaxially green, glabrous, usually ± complanate (rarely ± convex); abaxially white or glaucous, glabrous or with small branched brown-centred scales. Sori ± superficial, each with 2 (rarely 3) sporangia. Spores 34.8–45.1 µm long, 39.3–48.1 µm wide (20 populations).
The pouched or recurved ultimate segments with rounded apices and white or glaucous abaxial surfaces, sori mostly with 2 sporangia, and the intergrading mix of ovate and stellate scales on the abaxial surface of the β costae distinguish Gleichenia dicarpa from G. microphylla; the latter has complanate or recurved ultimate segments, with green abaxial surfaces and at least some apices cuspidate, sori mostly with >2 sporangia, and stellate scales with stiffly patent branches dominating the abaxial surface of the β costae. However, putative hybrids combining the character states of both species and/or with intermediate character states are widespread and fairly common.
The larger size of the fronds, pinnae with more than 2 pseudodichotomous forks, and accessory leaflets subtending the rachis buds distinguish most plants of Gleichenia dicarpa from G. alpina; definitively, G. dicarpa has stellate scales with patent branches (curled in Chatham Islands’ plants) on the β costae whereas G. alpina does not.
The superficial sori and mixture of ovate and stellate scales on the abaxial surface of the laminae separates Gleichenia dicarpa from G. inclusisora, which has sori embedded within pits in the lamina and ± orbicular scales.
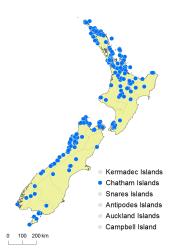
North Island: Northland, Auckland, Volcanic Plateau, Gisborne, Taranaki, Southern North Island.
South Island: Western Nelson, Sounds-Nelson, Marlborough, Westland, Canterbury, Otago, Fiordland, Southland.
Stewart Island, Chatham Islands.
Altitudinal range: 0–1350 m.
Gleichenia dicarpa is widespread from the far north of the North Island to Stewart Island, but it is uncommon to the east of the axial ranges of the North and South Islands, and apparently absent from much of the Fiordland coastline. It is the only Gleichenia on the Chatham Islands. It occurs from near sea level to 850 m above sea level in the North Island and most of the South Island, extending to 1000 m in Western Nelson Ecological Province and 1350 m in Canterbury Ecological Province.
Also Australia (Queensland, New South Wales, Victoria, Tasmania) and New Caledonia. The attribution of plants outside Australasia (e.g., Malesia) to Gleichenia dicarpa requires re-examination (see Notes).
Gleichenia dicarpa usually occurs in Leptospermum/Kunzea and other scrub and low forest, or swamps and other open habitats with wet ground, such as pakihi (wet, infertile heaths). It is often on road and track sides, and on poor substrates (e.g., clay, ultrabasic). It infrequently extends into forest (kauri, beech), and to drier ground. Gleichenia dicarpa tends to grow in more open and wetter sites than G. microphylla, but they often co-occur.
Morphological intermediates between Gleichenia dicarpa and G. microphylla are common (e.g., plants with ± complanate ultimate segments that are glaucous on their abaxial surface). These are potentially hybrids and that is how they are treated here (see Gleichenia ×punctulata).
Most putative hybrids with Gleichenia microphylla involve the morphological form of G. dicarpa that lacks both percurrent scales on the abaxial surface of the α costae and scales on the abaxial surface of the ultimate segments (see Notes below). But plants of G. dicarpa with percurrent scales on the abaxial surface of the α costae and scales on the abaxial surface of the ultimate segments from the northern North Island (see Notes) also seem to hybridise with G. microphylla (e.g., Kawerua, A.E. Wright s.n., AK 223063; Opua, L.R. Perrie 4814, WELT P022337).
Infrequent morphological intermediates between Gleichenia dicarpa and G. alpina suggest that this pair may also hybridise (e.g., near Blackball, L.R. Perrie 6376 & L.D. Shepherd, WELT P026765; near Haast, L.R. Perrie 6753 et al., WELT P026768).
n = 20 (Brownlie 1958, as Gleichenia circinata [sic])
As circumscribed here, Gleichenia dicarpa in New Zealand is morphologically and genetically variable. At least four groups can be recognised amongst New Zealand plants, but their distinctiveness needs further examination, and it is unclear which might be treated as separate taxa.
A) Plants with percurrent scales on the abaxial surface of the α costae and scales on the abaxial surface of the ultimate segments (e.g., Mount Somers, L.R. Perrie 3941 & L.D. Shepherd, WELT P021161; Pukerau, L.R. Perrie et al. 6296, WELT P026809; Key Summit, L.R. Perrie 5496 & L.D. Shepherd, WELT P026810). Groups C and D share these characteristics, but plants of group A are distinguished by the prominent, patently-branched stellate scales on the abaxial and adaxial surfaces of the β costae. Plants of group A have chloroplast DNA sequences closely related to plants of G. dicarpa from Australia and New Caledonia (D.J. Ohlsen et al. unpub.). Group A plants occur definitely in (southern) Westland, Canterbury, and Southland Ecological Provinces, possibly Stewart Island and in the eastern North Island (e.g., Kaweka Ranges, L.R. Perrie et al. 2983, WELT P020672), and perhaps sporadically elsewhere. Group A plants match the morphology of some plants of G. dicarpa in Australia and, given the close genetic relationship, are probably conspecific. However, Australian G. dicarpa might include more than one species as it is morphologically variable. For instance, the ultimate segments of some (but not all) Australian plants, including the lectotype of G. dicarpa, are strongly pouched with a pronounced lamina flange alongside the abaxial surface of the α costae. This feature does not occur in G. dicarpa from New Zealand, but is uniformly present in New Caledonia. The lectotype is otherwise similar to group A, except only in the smallest of three frond pieces are the stellate scales on the abaxial surface of the β costae darkly coloured with patent branches; in the two larger frond pieces, the corresponding indumentum is paler brown with twisted branches. The significance of this variation in Australia is unclear.
B) Plants with scales on the abaxial surface of the α costae that are not percurrent and ultimate segments with a glabrous abaxial surface (e.g., Heaphy Track, L.R. Perrie 4495, WELT P026785; Atarau, L.R. Perrie 6373 & L.D. Shepherd, WELT P026787). Plants of group B have chloroplast DNA sequences closely related to G. microphylla (Perrie et al. 2012) and G. mendellii (G.Schneid.) S.B.Andrews (D.J. Ohlsen et al. unpub.). Group B plants occur from the north of the North Island to Stewart Island; they are the most widespread and common G. dicarpa group in New Zealand, and the only form of G. dicarpa found in large parts of the country. The relationship of group B plants to G. mendellii from Australia requires investigation. Gleichenia mendellii shares the scale characteristics described above for group B plants, along with ultimate segments that are glaucous on their abaxial surface and relatively weakly pouched. However, the epitype of G. mendellii (Queensland, Russell Is., P.R. Sharpe 3130, BRI AQ0370733) differs in having a higher frequency of sori with more than two sporangia and sparser indumentum on the abaxial surface of the β costae. Based on their morphology, other Australian plants outside the currently accepted distribution of G. mendellii (south-eastern Queensland and north-eastern New South Wales; Chinnock & Bell 1998), and attributed to G. dicarpa, also need re-examination (e.g., Tasmania, Gordon River, A.T. Dobson 77029, CHR 313699).
C) Plants from the Chatham Islands (e.g., Chatham Island, P.J. de Lange CH919 & P.B. Heenan, AK 300719; Chatham Island, C.H. Hay s.n., 23 June 2007, WELT P026814). This is the only Gleichenia recorded from the Chatham Islands. Like those of group A, plants of group C have percurrent scales on the abaxial surface of the α costae and scales on the abaxial surface of the ultimate segments. Group C plants differ in that the stellate scales on the abaxial surface of the β costae have curled or twisted rather than stiffly patent branches. Chloroplast DNA sequences of group C plants are more closely related to group B plants (and G. microphylla) than to group A plants and Australian G. dicarpa (D.J. Ohlsen et al. unpub.).
D) Plants with percurrent scales on the abaxial surface of the α costae and scales on the abaxial surface of the ultimate segments (e.g., Karikari Peninsula, L.R. Perrie 4193, WELT P021155; near Taipa, L.R. Perrie et al. 6405, WELT P026777). The scales on the abaxial surface of the α costae have bodies usually less than 300 µm wide, such that they appear as a mass of tangled hairs (actually their cilia). This usually distinguishes them from groups A and C, whose wider scales on the abaxial surface of the α costae are quite evident. Additionally, group D plants have prominent, branched accessory leaflets around their rachis buds (like group B plants), but these are often lacking in group A and C plants (and are unbranched when present). Chloroplast DNA sequences of group D plants match those of group B plants (D.J. Ohlsen et al. unpub.). Plants of group D dominate G. dicarpa populations in Northland, are common in Auckland (together with group B plants), and appear to infrequently extend south to Taranaki and Volcanic Plateau Ecological Provinces.
In addition to its morphological and genetic variation, the taxonomy of Gleichenia dicarpa is further complicated by its nomenclature, particularly the long-confused G. circinnata Sw. (Christensen 1910; Holttum 1959; Brownsey et al. 1985). This was described by Swartz (1801), but only later said to be from Australia (Swartz 1806). It has been variously confused with both G. dicarpa and G. microphylla, both also described from Australian material, and both post-dating G. circinnata. The putative type material of G. circinnata, S P4151 and P4153 (online!), is fragmentary, but is clearly not G. microphylla. Holttum (1959), noting conflict with the protologue, argued that it was also not equivalent to G. dicarpa, but this requires re-evaluation in the context of a detailed review of the morphological variation in Australasian G. dicarpa. This should be done before making substantive nomenclatural changes, particularly given that G. dicarpa as currently circumscribed may comprise a complex of species in Australasia; in that context, G. circinnata and G. dicarpa may or may not be synonymous.
Gleichenia dicarpa has been attributed to New Caledonia and Philippines (e.g., Holttum 1959). However, the relationship of plants outside Australasia to Australian material representative of the type, and to the Malesian G. vulcanica Blume, requires re-evaluation.
The lectotype of Gleichenia hecistophylla A.Cunn. appears to belong to group B of G. dicarpa, but the other syntype belongs to group D (Perrie & Brownsey 2015).
The Tasmanian holotype of Calymella major Nakai has a morphology not found in New Zealand.