- ≡ Leskea sciuroides Hook., Musci Exot. 2, 175 (1819)
- ≡ Cladomnion sciuroides (Hook.) Hook.f. & Wilson in Wilson, Bot. Antarct. Voy. II (Fl. Nov.-Zel.) Part II, 100 (1854)
- = Glyphothecium muellerianum Hampe, Linnaea 30: 637 (1860)
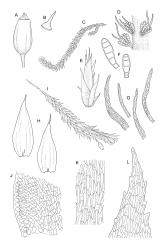
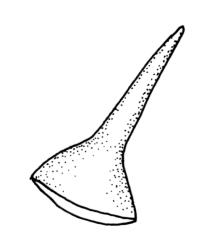
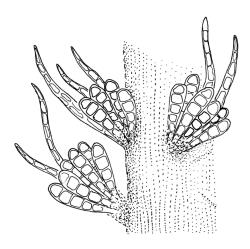
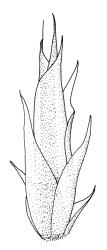
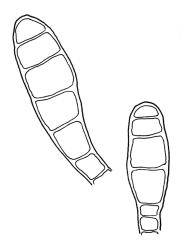
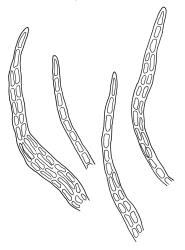
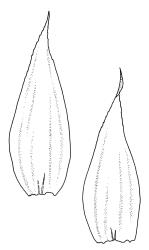
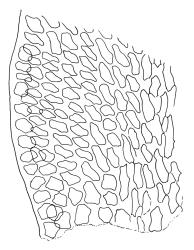
Plants rather pale green to brown-green, weakly lustrous, moderately robust, forming loose wefts, usually pendent and curved away from the substrate. Primary stems not seen, apparently lacking. Secondary (ascendant) stems 17–40(–80) mm, simple or branched by forking, in cross-section oval, with a few smooth, red-brown rhizoids at extreme base. Shoots not complanate, strongly curled away from the substrate at their tips. Leaves of secondary stems imbricate and erect-spreading when moist, little altered when dry, not complanate, often weakly secund, broadly ovate-lanceolate, gradually tapered to a narrowly acute and usually twisted apex, weakly and rather irregularly plicate (more conspicuously when dry), usually reflexed at mid leaf margins, symmetric, toothed above, mostly c. 2–3 × 0.8–1.1 mm; mid laminal cells irregularly rhombic, thick-walled, porose, c. 30–36 × 9 μm; alar cells differentiated, irregular in outline, oblate to ± quadrate, porose, compact, forming a small group, which merges gradually above with several rows of obliquely oriented and irregularly elongate cells. Costa short, double, and indistinct. Paraphyllia present and sometimes abundant, linear, mostly two cells wide at base and 180–400 μm long. Gemmae usually numerous among upper leaves, clavate, transversely septate, mostly 5–8-celled and c. 100–150 µm long.
Dioicous. Perichaetia scattered, appearing lateral on secondary stems, the inner leaves shouldered and tapered to a slender acumen, clasping the seta base. Male plants not dwarfed, with numerous scattered gemmiform and brown perigonia. Seta c. 5 mm; capsules erect and symmetric, ellipsoid, ribbed; exothecial cells rounded; stomata few, best seen in green capsules; annulus lacking; operculum c. ½ length of the capsule. Exostome teeth c. 360 µm, neither furrowed nor shouldered, unbordered, cross-striate below on abaxial surface, nearly smooth above; trabeculate but otherwise smooth on the adaxial surface; endostome arising from a pale membrane c. ½ the exostome height, with rudimentary segments. Calyptra cucullate, smooth. Spores single-celled, 15–18 (rarely –30) μm, papillose.
Hooker 1819–1820, pl. 175; Hattaway 1981, figs 1, 4–5, 7; Malcolm & Malcolm 2003, p. 30; Milne & Klazenga 2012, unnumbered fig. and photos; Seppelt et al. 2013, pl. 7.
Glyphothecium sciuroides is unlikely to be confused with other members of the Ptychomniaceae in N.Z. Hampeella alaris differs by being a more delicate and glossy plant, with distinctly reflexed leaf apices.
More compact forms of G. sciuroides bear considerable superficial similarity to Crosbya straminea and confusion does sometimes occur, especially in sterile material. Glyphothecium sciuroides has weak and double costae and unbordered leaves, in contrast to the strong single costa and distinct leaf border in C. straminea. The wider (mostly c. 1 mm) and less secund leaves of the Glyphothecium contrast with the narrower (mostly c. 0.5 mm) and distinctly secund leaves of the Crosbya. Glyphothecium stems bear paraphyllia, while those of Crosbya do not. Both species frequently bear capsules and then the ribbed capsules of Glyphothecium contrast with the smooth capsules of Crosbya.
NI: N Auckland (Puketi Forest, Waipoua Forest, Titirangi) including offshore islands (LB), S Auckland, Gisborne (numerous localities, including Lake Waikaremoana area), Hawke’s Bay, Taranaki, Wellington; SI: Nelson, Marlborough, Canterbury (numerous localities, all above c. 700 m elevation), Westland, Otago (localities mostly in eastern Otago), Southland.
Austral. Tasmania*, mainland Australia*, Argentina*. Reported from Chile by Hattaway (1981) and from P.N.G., several other Malesian islands, and elsewhere by Enroth (1991) and others. However, there is no consensus regarding the relationship of Australasian (N.Z., Tasmanian, and mainland Australian) populations of Glyphothecium to populations in Malesia (including P.N.G.) and Asia. Consequently, all published records from those regions are regarded here as inconclusive.
Mostly epiphytic on trunks and large branches, less often on stumps or boulders, and rarely on twigs. Occurring on a wide variety of woody species belonging to at least 16 genera. Commonly associated mosses include Cladomnion ericoides, Dicnemon spp., Hampeella alaris, Macromitrium longipes, Mesotus celatus, and Weymouthia cochlearifolia. Ranging from low elevations (c. 80 m at Otūmangu Landing on the Whanganui River, Wellington L.D.) to at least 1070 m (Mt Taranaki, Taranaki L.D.) on the North I. and from near sea level (at several localities including the Punakāiki River, Nelson L.D. and Pelorus Bridge Scenic Reserve, Marlborough L.D.) to >1100 m (St Arnaud Range, Nelson L.D.) on the South I. All Canterbury L.D. records are from elevations of >700 m, although there are several lowland records from Marlborough L.D., Glyphothecium is very frequent throughout Southland L.D. (>20 separate collections in CHR), making the absence of records from Stewart I. seem anomalous.
Spore characters are particularly difficult to observe in G. sciuroides. The measurements in the description are taken from spores in capsules with fallen opercula. Spores are often very few in number in such herbarium specimens. Some spores up to 30 µm diam. have been seen, and these correspond to the size Fleischer (quoted by Sainsbury 1955) mentioned for Javanese Glyphothecium. In addition to normally developed papillose spores, occasional thin-walled and collapsed spores occur. One instance has been seen (E.S. West, 24 Sep. 1938, WELT M003045) of gametophyte shoots emergent from capsules, apparently as the result of in situ germination.
The circumscription of this species and its consequent distribution outside of N.Z., Tasmania, and mainland Australia require clarification. The type locality is Tasmania, while the type locality of the synonym G. muellerianum is Victorian. Hattaway (1981) recorded it from Chile and western Argentina and indicated that it sometimes co-occurs there with G. gracile (Hampe) Broth. Two Patagonian specimens collected by P. Dusén (from Puerto Blest on Lago Nahuel Huapi in western Argentina, WELT M033715!, and Insula Guaitecas in Chile, WELT M033716!) have been confirmed as belonging to G. sciuroides.
Enroth (1991) recorded G. sciuroides from several high elevation localities in New Guinea, from whence van Zanten (1964) described G. pendulum. I am not convinced that the single New Guinean specimen I have examined is conspecific with N.Z. or Tasmanian material of G. sciuroides, but note that Enroth briefly discussed (citing an unpublished revision by Hattaway) the considerable variability of G. sciuroides on that island. Material of the Javanese G. pangerangense Fleisch. has not been studied.
According to Hattaway (1981), the South American G. gracile (Hampe) Broth. differs from the present species by having rectangular to linear alar cells, while he considered the alar cells of G. sciuroides to be quadrate to oblate. No reliably named material of G. gracile has been available for comparison; the absence of this species in N.Z. herbaria is difficult to explain.
There are discrepancies between the collection information in the protologue and that on the holotype in the BM. "D.R. Brown", given in the protologue, presumably refers to Robert Brown of Australian fame. The holotype does not bear the collector’s name, but bears a collection number: "8, L. sciuroides, Van Diemen’s Island" in Robert Brown’s script as well as a clear reference to Hooker’s (1819–1820) Musci Exotici pl. 175 (probably in the same script). The holotype is also labelled as Herbarium Hooker "H.1973". The two mounted stems comprising the holotype were illustrated in the protologue. There are other Tasmanian species published in the Musci Exotici (e.g. Bartramia affinis, Splachnum octoblepharum) that are cited as collected by either "D.R. Brown" or by "D. Brown".