- ≡ Glyphothecium alare Dixon & Sainsbury, J. Bot. 71: 246 (1933)
- = Hampeella pallens var. symmetrica Sainsbury, Rev. Bryol. Lichénol., n.s. 20: 95 (1951)
- ≡ Hampeella alaris var. symmetrica (Sainsbury) J.Milne & Klazenga, Austral. Mosses Online 31: 2 (2012)
The species description below deliberately repeats information in the generic description, reflecting my persistent doubts about a close relationship between these two species.
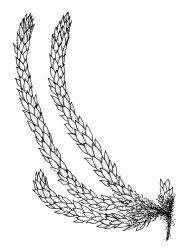
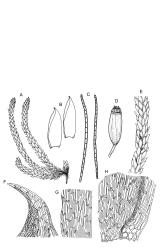
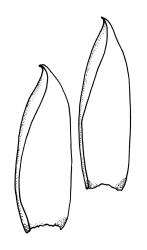
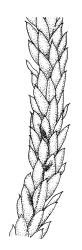
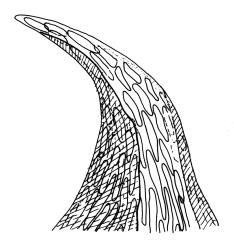
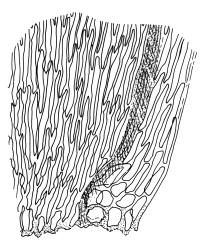
Plants pale yellow-green, lustrous, rather slender, forming loose wefts, usually weakly curled upwards. Primary stems weak and creeping or sometimes apparently absent. Secondary shoots arising in tufts, variable in length, c. 20–35 mm, simple or sparsely forked, ± julaceous (not complanate) below, tapered to a slender and weakly complanate apex, with stems in cross-section oval, lacking a central strand, with several layers of thick-walled, pigmented cortical cells, and with a few smooth red-brown rhizoids at base. Leaves of secondary shoots imbricate, erect-spreading, arranged in several weakly defined spiral ranks, elliptic and rather abruptly tapered to an acute, reflexed, and untwisted mucro, concave and with margins strongly inrolled above, smooth, symmetric, entire except for a few apical denticulations, c. 1.3–1.5 × 0.4 mm; those near stem apex nearly always more lanceolate, markedly less concave, and not reflexed at apex; mid laminal cells linear-rhombic, ± thin-walled, smooth, usually slightly porose, c. 60–90 × 4–5 μm; cells at insertion irregular, thick-walled, porose, pigmented, forming a ± distinct band across the leaf base; alar cells forming a small, well-defined group of c. 7–10 irregularly quadrate, thick-walled, and somewhat enlarged (c. 15–21 μm in greater dimension) cells. Costa short and double, indistinct, or lacking. Paraphyllia apparently absent (see below). Gemmae abundant or sparse among upper leaf axils, acicular and uniseriate, mostly 20–25 cells and 750–1200 μm long, arising from tufts of short rhizoid-like structures.
Dioicous. Perichaetia on short branches, scattered and appearing lateral on secondary shoots, inner leaves broadly ovate, acute, not shouldered, apparently enlarging after fertilisation and clasping the seta for up to half its length. Perigonia bud-like, scattered on well developed ♂ shoots. Setae 3–5(–6) mm, flexuose; capsules erect, symmetric, strongly sulcate when dry, c. 2.5 mm; exothecial cells firm-walled, rounded or somewhat polygonal; stomata few at extreme capsule base; annulus weakly developed, composed of 1(?) row of thin-walled cells; operculum as per genus, c. ½ the length of the capsule. Exostome teeth yellow, broadly lanceolate, not shouldered, c. 330 μm long, bordered, inserted just below the rim, with a ± zig-zag divisural line, not furrowed, transversely finely striate below on outer surface, densely trabeculate but otherwise nearly smooth on inner surface; endostome arising from a basal membrane c. ½ the height of the teeth, with nearly colourless and smooth segments extending c. ⅞ the height of the teeth and no cilia. Calyptra not seen, probably cucullate and smooth. Spores extremely variable, round or elliptic, green, papillose, mostly 12–45 μm, but often appearing collapsed in dried material and sometimes larger.
Sainsbury 1955, pl. 54, fig. 2; Malcolm & Malcolm 2003, p. 32; Milne & Klazenga 2012, unnumbered fig. and photos; Seppelt et al. 2013, pl. 8.
Confusion sometimes occurs between H. alaris and Sauloma tenella due to both having similarly shaped leaves with reflexed apices. Hampeella alaris invariably has an erect habit, while the paler S. tenella sometimes forms prostrate or even pendent mats. The leaves of H. alaris lack the longitudinal pleat that occurs in Sauloma. The upper laminal cells in the present species are c. 60–90 × 4–5 μm, while those in S. tenella are mostly 75–135 × 12–15 μm.
NI: S Auckland, Gisborne, Taranaki, Wellington (Mt Tongariro, Ruapehu, Tararua Range). SI: Nelson, Marlborough (Mt Stokes, Pelorus Bridge Scenic Reserve), Canterbury, Westland, Otago, Southland; St.
Australasian. Tasmania*. Reported from Victoria by Vollebergh (1986) and by Milne & Klazenga (2012).
In forest and usually on small branches and twigs of a wide range of woody plant species, less often on trunks. Host plants include at least 11 different woody genera. Commonly associated mosses include Cladomnion ericoides, Crosbya straminea, Glyphothecium sciuroides, Leptostomum inclinans, Macromitrium longipes, and Weymouthia mollis. Ranging from c. 215 (Mayor I., S Auckland L.D.) to at least 1070 m (Panekiri Range, Gisborne L.D.) on the North I. and from c. 30 (Lyell Creek, Nelson L.D. to 1050 m on the South I).
This species is uncommon in the eastern, drier portions of the South I. R. Seppelt (pers. comm., 13 March 2002) informs me that it is restricted to wetter and more western portions of Tasmania.
When occurring on small branches this species grows as irregular rosettes of ± erect and usually curved secondary shoots c. 20–35 mm in height. The axillary gemmae can be very abundant amongst the upper leaves and can occasionally be seen with a hand-lens. When leaves are stripped from the stem, uniseriate and filamentous hairs (in addition to the brood bodies) are usually visible associated with the leaf base. These are mostly c. 200 μm and 5–6 cells long. They are interpreted here as axillary hairs rather than as paraphyllia. What appear to be lanceolate pseudoparaphyllia, c. 100 μm long and 2 cells wide at base are present at the base of perichaetia, but these have not been observed at the base of vegetative branches. I am, like Sainsbury (1955, p. 345), uncertain concerning the size and morphology of the spores, and in most fruiting collections have been unable to satisfactorily observe them. In at least one herbarium collection (W. Martin 13 Feb.1951 ex Doubtful Sd., CHR 558927) the spores are distinctly dimorphic within a single capsule, with some 39–54 μm and others c. 21–24 μm and somewhat collapsed. It is unclear whether such dimorphic spores are the normal condition in this species or whether the smaller spores are merely undeveloped in this material.
Aberrant material has been collected at Marawaiwai Scenic Reserve (near Ōpōtiki, Gisborne L.D.) by P. Beveridge LU-43 (WELT M040630). This material has leaves on the lower stem portions arranged in spiral ranks and with weakly reflexed apical mucros. There is a small group of rounded-subquadrate alar cells present, forming a small decurrency. The axillary gemmae here are mostly 400–640 µm and 15–22 cells long. These are shorter (in overall length) than usual for the gemmae of H. alaris and scarcely overlapping in terms of the cell number. While in some respects the Marawaiwai material is suggestive of H. pallens, it has been referred to H. alaris. Beveridge’s Marawaiwai collection compares very well to type material of the previously synonymised Hampeella pallens var. symmetrica Sainsbury.
All the material of H. alaris appears to be dioicous, Sainsbury’s (1955, p. 345) observation that the sexuality is "apparently variable" notwithstanding.
The generic placement of this species is problematic. When it was originally described in Glyphothecium, Dixon & Sainsbury (1933) expressed reservations concerning its belonging in that genus. The lack of paraphyllia, the acicular nature of the brood bodies, the thin-walled, scarcely porose and ± rhombic laminal cells, the strongly defined alar group of enlarged but thick-walled cells, the absence of oblique cells above the alar group, the lack of leaf plications, and the presence of well-developed endostomal segments all argue strongly against its retention in Glyphothecium.
Dixon & Sainsbury (1933) also suggested that this species might be allied to Clastobryum, a small genus of East Asian distribution in the Sematophyllaceae. The apiculate and reflexed nature of the vegetative leaves in Hampeella alaris argue against its placement in that genus while such leaf apices are found elsewhere, both in the Ptychomniaceae (e.g., Cladomnion) and in the Sematophyllaceae. The erect, symmetric and ribbed capsules, the epiphytic habitat, the presence of weakly developed primary stems giving rise to tufts of upwardly curved secondary shoots, and the presence of a band of thick-walled and strongly porose cells at the base of the leaves are all features that argue in favour of retention in Ptychomniaceae.
In 1951 Sainsbury transferred G. alare to an expanded Hampeella. In his enumeration of the species’ outstanding gametophytic features Sainsbury noted the polymorphic and non-plicate nature of the vegetative leaves ("which in the main part of the stem are oblong-oval, very concave, cucullate and cuspidate, with the apex strongly recurved, but which in the upper part of the stem become much narrower, less concave, not cucullate, gradually acuminate and bluntly pointed"), filamentous axillary brood bodies, the absence of paraphyllia, linear-rhomboid, weakly porose laminal cells, and large, incrassate, and coloured alar cells.
Sainsbury’s (1951) placement of this species in Hampeella is followed here with reservations. Scott & Stone (1976, p. 362) have expressed similar doubts, without citing their reasons. The most notable differences between the two N.Z. species of Hampeella are found in the exostome teeth. These lack a distinct abaxial furrow (and associated shoulder) in H. alaris, while those of H. pallens have a well-developed median adaxial furrow and are shouldered. Furrowed exostome teeth are widespread in the Ptychomniaceae. Also, the leaves on the lower stems of H. alaris are symmetric, apiculate, strongly concave, arranged in weakly defined spiral ranks, and reflexed at the apex, whereas those of H. pallens are asymmetric, non-apiculate, nearly flat (except at the extreme basal margin), and not reflexed at the apex. Further study of H. alaris may show it to deserve a new genus of its own.
Hampeella pallens var. symmetrica Sainsbury is treated here as a synonym (and unworthy of segregation at any taxonomic level) of H. alaris. The recognition of Sainsbury’s var. symmetrica as a variety within H. alaris, as has been proposed by Milne & Klazenga (2012), is not accepted here.