- = Zygodon conoideus var. elongatus Hook. & Grev., Edinburgh J. Sci. 1: 132 (1824) – as Zygodon conoideum var. β elongata
- = Zygodon gracilicaulis Müll.Hal., Hedwigia 37: 135 (1898)
- = Zygodon nanus Müll.Hal., Hedwigia 37: 135 (1898)
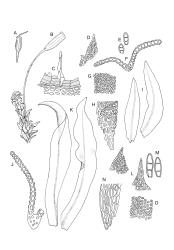
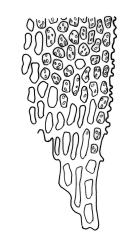
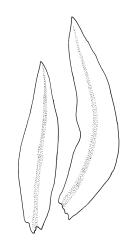
Plants medium-sized, 5–25(–55) mm tall, in dense tufts or turves; green to yellow-green above, red-brown to brown below. Stems frequently branched (often by innovation), in cross section with small, incrassate cortical cells and lacking a central strand; rhizoids well-developed and extending well up the stem, much branched, red-brown, finely papillose. Leaves erect appressed and imbricate, loosely twisted round the stem, homomallous, or strongly contorted when dry, erect-spreading when moist, lanceolate or linear lanceolate, acute, entire or slightly crenulate from projecting papillae, sometimes with a few small denticulations near the apex formed by protruding cells, weakly decurrent, weakly keeled (more prominently in lower portions), often ± undulate, plane at margins, 0.6–1.8 × 0.2–0.3 mm (under cover slip); upper laminal cells irregularly rounded, almost isodiametric, 4–10(–15) µm in greater diam., with moderately thick walls, with 4–8 short, simple, round papillae on each surface; apical cells usually papillose, rarely smooth; basal cells rounded rectangular or rectangular, moderately thick walled, smooth, mostly 15–25 × 7–9 μm, extending only a short distance up the leaf and grading rapidly into shorter and papillose cells; alar cells not differentiated. Costa ending below leaf apex, with cells on both surfaces elongate, protruding and smooth or papillose in upper portions on the abaxial leaf surface, lustrous when dry. Gemmae small, rarely seen, clavate or ellipsoid, with hyaline transverse walls, usually 3-celled and 45–60 μm.
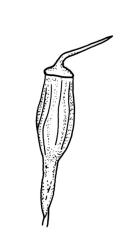
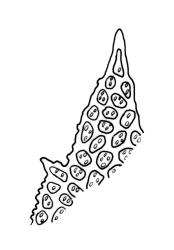
Dioicous. Male and female plants of similar size. Perichaetia terminal, usually overtopped by 1 or more innovations, with leaves longer and with the basal cells longer and extending further up the leaf than in vegetative leaves. Perigonia terminal and gemmiform, often appearing lateral by innovation, the inner bracts broadly ovate, obtuse, and brown. Setae 2.5–10 mm, dextrorse above, pale yellow-brown; capsules exserted, ± ellipsoid when moist, becoming pear-shaped, oblong or cylindric and deeply 8-furrowed when dry, orange-brown to light brown, 1.0–1.5 mm; exothecial cells with longitudinal walls much thicker than transverse walls, arranged in alternating bands of thick- and thin-walled cells; stomata restricted to neck, superficial; annulus not seen; operculum obliquely long-rostrate from a conic base, mostly c. ⅓ the length of the capsule. Peristome double; exostome of 8 short, sometimes reduced teeth or absent; endostome of 8 hyaline, smooth, more or less reduced segments. Calyptra cucullate, smooth. Spores globose, insulate-papillose, green or yellow-brown, 13–17(–20) µm.
Malta 1926, figs 39–40; Lewinsky 1990, figs 131–153; Calabrese 2006, figs 17, 18 a–b; Seppelt 2004, fig. 84.
The majority of collections of Z. intermedius, when dry, are yellow-green with well-developed red-brown rhizoids, rather broad, homomallous leaves with a conspicuous costa that protrudes and is lustrous abaxially, and dull leaf lamina that give this species (and its close ally Z. hookeri) a characteristic look. While microscopic examination is usually required to confidently distinguish it from Z. hookeri, the generally smaller stature of Z. intermedius, its narrower leaves, and shorter setae and capsules permit at least provisional field recognition of this widespread species. Zygodon intermedius has a wider elevational range and is more common below c. 500 m than its more robust congener. Perigonia are usually easily demonstrated in Z. intermedius, but are absent in the synoicous Z. hookeri. Despite its dioicous sexuality, Z. intermedius frequently produces capsules. Gemmae in Z. intermedius appear to be produced only in poorly developed plants and they have little practical value in the recognition of this species.
Apart from confusion with Z. hookeri, Z. intermedius is most likely confused with members of the pottiaceous genera Gymnostomum and Anoectangium. Zygodon intermedius differs in many ways from Gymnostomum calcareum. The Zygodon is a coarser plant in which the leaves are both longer and wider (0.6–1.8 × 0.2–0.3 mm vs 0.7–1.0 × <0.2 mm in Gymnostomum). The costa in Zygodon is either smooth or papillose abaxially in its upper portions only, while that of G. calcareum is conspicuously abaxially papillose nearly to its base and also papillose on its adaxial surface. The acute leaf apices of Z. intermedius contrast with the often obtuse or rounded leaf apices of G. calcareum. The setae in Z. intermedius are stouter and the capsules both strongly ribbed and stouter (>0.5 mm diam.) compared to the cylindric, smooth, and slender (c. 0.2–0.3 mm diam.) capsules of G. calcareum. The often epiphytic occurrence of the Zygodon contrasts with the exclusively epilithic or terrestrial substrate of G. calcareum.
Confident distinction of Z. intermedius from Anoectangium aestivum is more difficult. Although the general stature of the plants and the leaf size can be similar, the leaves of Z. intermedius (and Zygodon spp. generally) are less keeled than those of the Anoectangium. The costa in the Zygodon is smooth abaxially in its lower portions and ends short of the leaf apex, while the costa of A. aestivum is papillose abaxially for its entire length and usually percurrent as a short cusp. The gametangia of the Zygodon are terminal (but often appear lateral due to innovation) while those of A. aestivum (and the genus Anoectangium as a whole) are lateral. The strongly ribbed capsules in Z. intermedius and its often epiphytic substrate contrast with the smooth, ± oblong capsules and the usually epilithic substrate of the Anoectangium.
When on rock, Z. intermedius frequently grows with Amphidium spp. and in some ways has a similar appearance. However, the vegetative leaves of Z. intermedius are much shorter (c. 0.6–1.8 mm vs 2.5–4.0 mm in our species of Amphidium) and less contorted with dry. The laminal cells of Z. intermedius are isodiametric with relatively high papillae only over the cell lumina, while the laminal cells of both our species of Amphidium tend to be oblate and have lower papillae extending over the cell lumina and adjacent cell walls. Elongate cuticular striations often occur in Amphidium. When fruiting the exserted ± ellipsoid capsules borne on elongate setae in Z. intermedius contrast with the urceolate and emergent to short-exserted capsules borne on short setae in Amphidium.
K; NI: N Auckland, including offshore islands (PK, LB, RT), S Auckland, Gisborne, Hawke’s Bay, Taranaki, Wellington; SI: Nelson, Marlborough, Canterbury, Westland, Otago, Southland; St; Ch; A; C; M. The N.Z. distribution was mapped by Lewinsky (1990, fig. 155).
Anomalous. Mainland Australia*. Reported from Tasmania and from a single locality in northern Queensland by Lewinsky (1990, fig. 154). Reported from South America, Africa and Asia by Malta (1926).
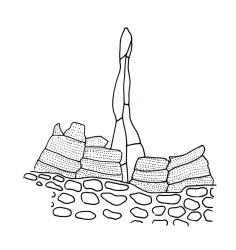
Zygodon intermedius grows most commonly as an epiphyte, often on the undersides of branches and leaning trunks, but it also occurs commonly on rock and logs through a wide elevational range. Epilithic occurrences (often, but not always, on cation-rich rocks) are more frequent at higher elevations. Of the identifiably unique collections in CHR, 145 were epiphytic, 90 epilithic/terrestrial, 19 on logs/stumps, and 36 lack substrate information. There appears to be a tendency for this species to occur more frequently on rock in the southern part of the South I.
This species occurs epiphytically on a wide range of indigenous trees and shrubs, including podocarps and some tree ferns as well as the introduced genera Cupressus, Populus, and Salix. This species is very tolerant of a wide range of humidity and tolerates at least moderate levels of salt spray. It is one of the most widely distributed mosses in the country and, accordingly, co-occurs with a wide range of both epiphytic and terrestrial bryophyte species. On the North I. it ranges from c. 20 m (Dacre Point, N Auckland L.D.) to c. 1200 m (Mt Ruapehu, Wellington L.D.) and on the South I. from near sea level (Akatore, Otago L.D.) to 1650 m (Mt Arthur, Nelson L.D.).
Lewinsky (1990) discussed at some length the provenance of the type collection of Z. intermedius and concluded that it was collected by A. Menzies at Dusky Bay, Southland L.D. According to Lewinsky (1990), the type specimen for Z. intermedius is also the type for Z. conoideus var. elongatus and is probably the same material on which Z. angustifolius Hook. & Grev. was based.