- = Blindia egmontensis Sainsbury, Rev. Bryol. Lichénol., n.s. 18: 111 (1949)
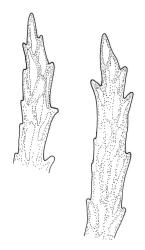
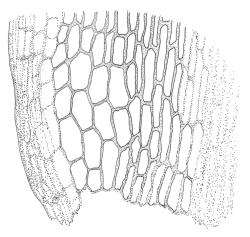
Plants gold-brown or yellow-green above, darker below, glossy and silky. Stems commonly 10–30 mm, wiry, much-branched by forking (and often by innovation in male plants), flexuose, with cortical cells thick-walled in c. 2 layers and a large central strand, with adherent fragments of stripped leaf bases not conspicuous. Leaves falcate-secund to circinate when dry, similar when moist, lanceolate-subulate, not penicillate, clasping and ± auriculate at base, evenly tapered to a fine subula ± equal the lamina (under stereoscope), acute, denticulate near apex, subtubulose below, c. 3.5–4.5(–6.0) mm; upper laminal cells (c. ⅓ above base) linear and ± straight, unistratose, strongly incrassate (the walls ± equal the lumina), c. 39–50(–60) × c. 3–5 µm, but slightly expanded at cell ends; lower laminal cells somewhat longer, either straight or ± sinuose, not porose; alar cells variably differentiated and often more developed on one side of the leaf base, usually forming a well-defined, weakly auriculate, and red group of moderately inflated, ± oblong, and ± thin-walled cells which are isolated from the costa by elongate cells. Costa c. 30–40 µm wide, narrow and ill-defined in lower leaf (c. ⅓ above base), becoming ± broader but faint at leaf base, filling the subula, in cross-section with stereids and guide cells poorly differentiated.
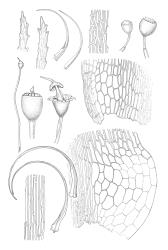
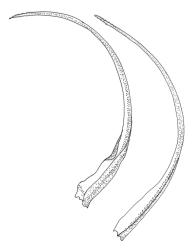
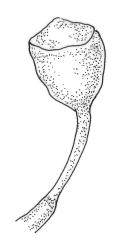
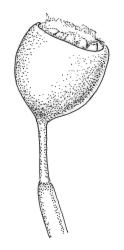
Dioicous or autoicous. Perichaetia terminating the stem but often overtopped by innovations; perichaetial leaves from a broadly oblong and sheathing base, abruptly shouldered and narrowed to a long and flexuose subula. Perigonia apparently terminal and overtopped by innovations, on autoicous plants occurring both above and below the perichaetia, the inner bracts <1.0 mm, lacking a subula, broadly ovate. Setae c. 0.2–0.6(–0.7) mm (above the foot which is c. 0.5 mm long), erect and ± straight, apparently untwisted; capsules broadly urceolate or obovate and wide-mouthed when dry, becoming hemispheric when moist, immersed, yellow-brown, not pachydermous, 0.6–0.8 × 0.6–0.7 mm; exothecial cells mostly oblong but somewhat irregular, incrassate and weakly collenchymatous, with several rows isodiametric or oblate at rim; stomata not seen; operculum low-conic or mammillate from convex base; columella sometimes adherent to and falling with the operculum. Peristome well-developed, yellow-brown, the 16 teeth broadly lanceolate-truncate, cribose and irregularly divided above, extending c. 150–180 µm beyond the rim, the outer surface nearly smooth; preperistome lacking. Calyptra as per genus. Spores c. 18–24 µm, green, papillose.
Sainsbury 1955, pl. 13, fig. 2; Bartlett & Vitt 1986, figs. 65–73, 153, 158.
NI: Gisborne (Mt Hikurangi), Taranaki (Mt Egmont); SI: Nelson, Marlborough (Mt Fishtail), Canterbury, Westland (Sewell Peak, Upper Ōtira Valley, Hōhonu Range), Otago (Old Man Range, Rock and Pillar Range, Siberia Valley), Southland (Hollyford River, Mt Burns, Gertrude Valley). Reported from A and C by Bartlett & Vitt (1986).
Australasian. Tasmania*.
On irrigated rock, especially at the margins of cascading mountain streams or in the beds of intermittent watercourses. Also occurring on high-altitude rock faces and in seepages associated with late snow beds (here often with Andreaea australis). This species is virtually never found submerged, and occurs on a range of rock types, including gneiss, greywacke, and sandstone. One collection has been seen from dolomite (Mt Burnett, Nelson L.D.). On North I. material has only been seen from above 1200 m; on South I. ranging from c. 850 (Ōtira Valley) to c. 1900 m elevation (Malte Brun, Canterbury L.D.) Associated species can include Andreaea acutifolia, A. australis, A. nitida, Blindia magellanica, B. robusta, Dicranoweisia antarctica, Racomitrium crispulum s.l., R. crumianum, R. ptychophyllum, Rhacocarpus purpurascens, and Schizymenium bryoides as well as the hepatics Jamesoniella colorata and Nothogymnomitrion erosum.
Bartlett & Vitt (1986) described this species as abundant on Mt Egmont, at Arthur's Pass, and in Fiordland. It is widespread and abundant at higher altitudes in the Paparoa Range (Nelson and Westland L.D.) and probably more common in wetter parts of the South I. than collections suggest. It is distributed primarily in areas of high rainfall.
Sainsbury (1949) described B. martinii and B. egmontensis in the same paper, with the latter termed "in most respects a miniature" of the former. The range of variation of plant stature, alar cell development, and upper leaf cell length is considerable in populations of B. martinii and Bartlett & Vitt (1986) placement of B. egmontensis in synonymy is followed here.
The plants usually have a decidedly "silky" appearance, although material from the southern South I. appears less so.
Blindia martinii is the only species of Blindia from N.Z. (and likely anywhere) with low-conic to mammillate and non-rostrate opercula. Its deeply immersed capsules with well-developed peristomes, along with yellow-green or golden-brown falcate-secund leaves that have denticulate upper leaf margins (best seen in leaves from near the stem apex), and differentiated alar cells further distinguish this species. According to Bartlett & Vitt (1986) "no other species of Blindia has such strongly falcate-secund leaves (moist or dry) and such deeply immersed, peristomate capsules".
The sexuality of plants of B. martinii is difficult to demonstrate. While the species appears to be mostly dioicous, the much-branched male and female stems are often closely intertwined. Bartlett & Vitt (1986, p. 223) indicate the sexuality to be either "dioicous or autoicous". I have seen definite autoicous plants (CHR 438172 from Scarlett Range, Nelson L.D.) and have also seen material that I considered to be dioicous (CHR 380530 from Arthur’s Pass).
The single specimen seen from Tasmania (A.J. Fife & R.S. Tangney 8944, CHR 475612; HO 544022) differs from representative N.Z. material in a number of ways including setae length (0.7–1.0 mm), larger capsules (0.80–0.95 × 0.9–1.0 mm), spore diameter (c. 24–42 µm vs 18–24 µm in N.Z. material), and central strand development (apparently lacking vs large and conspicuous in N.Z. material). The Tasmanian plants are not glossy, but relative lack of gloss is a feature of B. martinii from the southern South I. While an argument could be made for describing the Tasmanian material at some taxonomic level, I consider that its segregation from B. martinii would be unwarranted. Blindia martinii is not recorded from Tasmania by Dalton et al. (1991) and additional collections would be useful.