- ≡ Dicranum clavatum R.Br. in Schwägrichen, Sp. Musc. Frond. Suppl. 3(2), 255a (1829)
- = Campylopus appressifolius Mitt. in Hooker, Handb. New Zealand Fl. 414 (1867)
- = Campylopus arcuatus R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 474 (1897)
- = Campylopus arenarius R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 475 (1897)
- = Campylopus cylindrothecum R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 473 (1897)
- = Campylopus ellipticothecum R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 473 (1897)
- = Campylopus otaramaii R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 474 (1897)
- = Campylopus rarus R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 470 (1897)
- = Dicranum sulphureoflavus Müll.Hal., Hedwigia 36: 352 (1897) – as sulphureo-flavus
- ≡ Campylopus sulphureoflavus (Müll.Hal.) Paris, Index Bryol. Suppl. 98 (1900) – as sulphureo-flavus
- = Campylopus traillii R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 468 (1897)
- = Campylopus walkerii R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 469 (1897)
- = Campylopus persimplex Müll.Hal., Abh. Naturwiss. Vereins Bremen 16: 496 (1900)
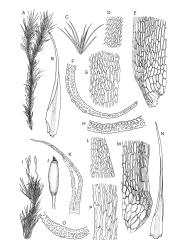
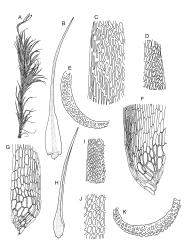
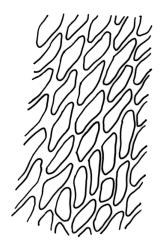
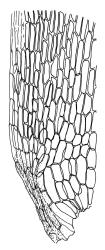
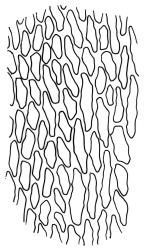
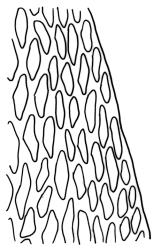
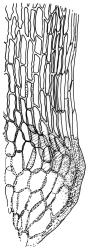
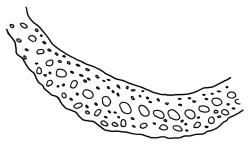
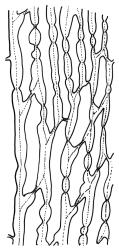
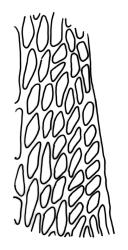
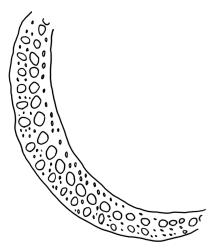
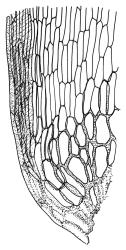
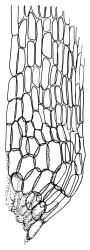
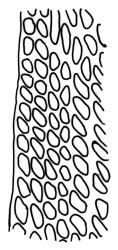
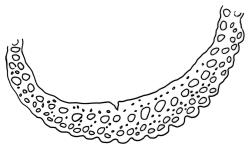
Plants yellow- or brown-green to nearly black, usually brown below, often forming extensive turves. Stems (10–)20–55(–90) mm, not or sparsely branched, in cross-section with a central strand and 3–5 layers of thick-walled cortical cells, apparently lacking a hyaloderm; rhizoids red or often becoming nearly white, smooth, densely covering the lower stem and some arising from the abaxial surface of the costae. Leaves erect spreading when moist, somewhat more erect but otherwise not altered when dry, evenly distributed on lower stem, often aggregated and more spreading at stem apex in both male and female plants (but not comose), subulate-lanceolate and evenly tapered to a subulate apex, strongly concave or subtubulose, concolorous or with a hyaline hair-point, denticulate at apex, entire below, very weakly bordered for ⅓ or less of leaf length, mostly 4.5–6.0 × 0.55–0.7 mm (flattened); basal laminal cells elongate-rectangular, forming an area of variable size, mostly 30–65 µm, thin-walled, not porose, weakly pigmented, lacking a sharply defined upper limit; laminal cells of upper base (c. 650–1100 µm above leaf base, depending on leaf length) extremely variable in form, wall thickness and porosity, usually rhombic or elongate-rhombic, ± obliquely oriented, and usually ± sinuate; occasionally regular and short-rectangular; cells adjacent to costa usually more elongate and porose; upper laminal cells (at c. mid leaf and upwards) mostly elongate-rhombic or rounded-rectangular but often irregularly elongate and incrassate, variably porose, often strongly so; alar cells strongly inflated and pigmented, forming a large, slightly auriculate group, rarely weakly differentiated; marginal cells scarcely differentiated. Costa c. 285–350 wide and 50–60% of the leaf base width, usually difficult to see at mid leaf due to concavity, filling the upper subula and appearing excurrent, lacking lateral spurs, usually with rhizoids arising from lower abaxial surface, in both abaxial and adaxial surface view the cells linear; in cross-section (mid leaf) with a single central layer of guide cells, 1 layer of exposed adaxial stereids, and 2(–3) layers of abaxial stereids, which are usually interspersed with larger cells (± same diameter as the guide cells); the abaxial surface smooth, undulate or with low and ill-defined single-celled ridges; in cross-section (at leaf base) the adaxial layer of exposed cells enlarged. Propagula often present in sterile plants, consisting of deciduous, stellate shoot fragments.
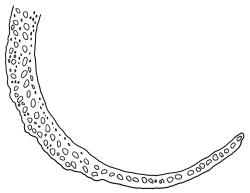
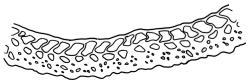
Dioicous. Perichaetial leaves weakly sheathing, with a more distinct ± oblong base but otherwise not differentiated. Setae cygneous, 7–10 mm, dark brown to nearly black; capsules subcylindric, erect, non-strumose, smooth or nearly so when dry, scabrid at base, c. 2 mm; operculum rostrate, c. half the length of the capsule. Peristome teeth orange below, pale above, coarsely and irregularly lirate throughout, forked nearly to base. Calyptra fringed at base. Spores 9–15 µm, smooth.
Catcheside 1980, fig. 42; Frahm 1984, figs. 9–14; Frahm 1987, fig. 6; Meagher & Fuhrer 2003, p. 107.
Campylopus clavatus can sometimes be difficult to distinguish from C. purpureocaulis. Several differences are cited under the discussion of the latter species, including substrate preferences, propagulae form, upper laminal cells features (often the easiest feature to observe), as well as sporophytic differences. Occasionally sectioning of the costa is required to separate these two species. Campylopus purpureocaulis is the only other N.Z. species of the genus having stereids exposed on the adaxial surface at mid leaf. In C. clavatus these adaxial stereids are in a single layer, while in C. purpureocaulis there are two to several layers of adaxial stereids. In C. clavatus abaxial costal ridges at mid leaf are mostly absent, while in C. purpureocaulis crenulations or low ridges are often present (see Image: Image\2TFZ, fig. E, and Malcolm & Malcolm 2006, p. 286). The two also differ in a cross-section of the costa taken just above the upper limit of the alar cells. In C. clavatus such a basal costal cross-section shows the exposed adaxial cells to be inflated and thin-walled (hyalocysts, see Catcheside 1980, fig. 42d), while in C. purpureocaulis the exposed adaxial cells are small and incrassate.
NI: K; N Auckland including offshore islands (TK, HC, LB, GB), S Auckland, Gisborne, Hawke’s Bay, Taranaki, Wellington; S: Nelson, Marlborough, Canterbury, Westland, Otago, Southland; St; Ch; A; Ant; C; M.
Australasian or Austral. Tasmania*, mainland Australia*. Reported from Chile by Robinson (1975).
Occupying diverse terrestrial habitats, on soil of a wide range of texture and nutrient content and tolerant of a range of both moisture and insolation. Occasionally on thermally heated soil; also on rock and occasionally on logs. Virtually ubiquitous throughout N.Z. From near sea level (Waikuku Flat near North Cape, N Auckland L.D.) to at least 1550 m (Bruce Road, Wellington L.D.) on the North I. and at least 1550 m (Corner Peaks, Southland L.D.) on the South I.
Campylopus clavatus, as interpreted here, is a highly variable species in many respects, including overall habit, degree of hair-point development, the extent of the basal laminal cells, and the form of the laminal cells. The extreme variability of the species, in particular in leaf characters, makes this species difficult to delimit satisfactorily and is reflected in the large number of taxonomic synonyms.
In addition to the synonyms given above, Robinson (1975) considered C. persimplex Müll.Hal. [Abh. Naturwiss. Vereins Bremen 16(3): 496 (1900)] to be C. clavatus. The type is H. Schauinsland 226 from French Pass (Nelson L.D.). It has not been seen but might be sought in the Bremen or the Helsinki herbarium.
When plants are fertile, the subcylindric, non-sulcate capsules with scabrid necks and peristome teeth, which are split nearly to their base and coarsely lirate, clearly distinguish this species from all its N.Z. congeners. The scabrous capsule neck and deeply divided peristome teeth (and their coarse, non-striolate ornamentation) are among the features used by Frahm (1984) to define the subgenus Thysanomitrion, which has only the single representative in N.Z.
The relationship between environmental factors and the morphological variability of this species, and especially the form and porosity of the laminal cells, is poorly understood. It is possible that a detailed morphometric or molecular study might permit the recognition of some of the variants of this species, but to recognise them at the present state of knowledge would be of little value. As in many unrelated moss species, very dark, sometimes ± black plants are associated with irrigated habitats.
Both male and female plants usually have the stem apices expanded and terminated by large numbers of antheridia or archegonia. The leaves subtending the sex organs, however, differ little in size from leaves on the non-expanded portion of the stem, and hence the plants cannot be considered comose. Sterile plants are uniformly foliate and not expanded apically. The frequent production of stellate shoot fragments facilitates recognition of sterile material. Also the rhizoids here often assume a white colour on the lower portions of the stem; when present a pale mat of rhizoids is unique among N.Z. Campylopus species.
In one of the most distinctive and widespread growth forms of C. clavatus (the so-called C. appressifolius), the leaves are markedly erect-appressed and stems are elongate and expanded apically only in gametangial plants. These features are correlated with the microscopic features of incrassate, strongly porose, and relatively elongate upper laminal cells that extend basally nearly to the alar cells. There is a tendency for such material to bear low and poorly developed abaxial costal ridges at mid leaf. While a case could be made for recognition of the appressifolius growth form at the varietal rank, this is not done here as many populations show a morphological transition from representative C. clavatus to the appressifolius growth form. Such material (e.g., G. Brownlie 305 from Charleston, Nelson L.D., CHR 425962) often has a small group of short, rectangular cells present between the basal laminal cells and the relatively elongate and porose upper laminal cells and weakly developed abaxial costal ridges. Dixon’s (1923, p. 84) observation that the appressifolius growth form (cited as C. appressifolius) has a costa "usually less than half width of the leaf-base" and often long hair points does not agree with my own.
Frahm’s (1984, p. 586) statement that all members of the subgenus Thysanomitrion have a hyalodermis is not supported by my observations on C. clavatus.
Robinson (1975) suggested that C. clavatus is closely related to C. richardii Brid. of tropical America, a statement that I strongly endorse. This synonymy is not made here partly because only limited material of South American and West Indian C. richardii is available for comparison. The available material, however, suggests a similar wide range of morphological variability in the American taxon. Although some collections of C. richardii have elongate, thick-walled, and porose laminal cells extending basally to the alar group (cf. Frahm 1994, fig. 97), in other collections a small, poorly defined area of elongate, thinner-walled, non-porose cells occurs immediately above the alar cells; this is similar to the condition in the majority of N.Z. material. I have seen Colombian and Antiguan material of C. richardii, which, if collected in N.Z., I would unhesitatingly refer to C. clavatus.