- = Campylopus arboricola Cardot & Dixon in Dixon, Bull. New Zealand Inst. 3: 90 (1923)
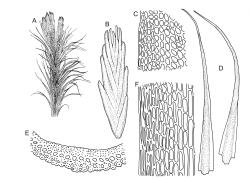
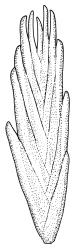
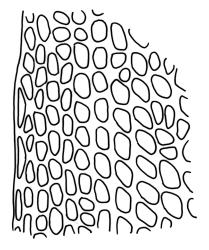
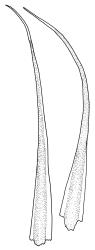
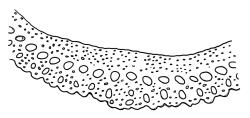
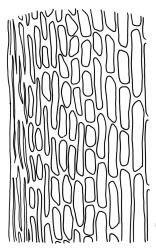
Plants bright yellow-green above, brick-red below due to dense rhizoids, forming tufts or turves, mostly on wood. Stems 10–c. 50 mm, mostly branched by innovation below sex organs or below a terminal cluster of brood bodies, in cross-section with a central strand, and one to several layers of thick-walled cortical cells, no hyaloderm; rhizoids densely covering the lower stems, brick-red and strongly contorted. Leaves erect-spreading or weakly secund when moist, more erect but little altered when dry, often ± comose immediately below masses of distichous propagula (often mixed with sex organs), narrowly lanceolate, not or only slightly narrowed at base, concave, finely acuminate at apex, with a well-developed hyaline hair-point (at least in upper leaves) or occasionally with concolorous tips, entire except for a few projecting cells near apex of hair-point, with a very weak border which does not extend above the leaf base, c. (2–)3–6 × c. 0.5–0.7 mm; upper laminal cells (mid leaf and above) not or slightly obliquely oriented, small and irregular, at least some subquadrate and some oblate, firm-walled, not porose, mostly (6–)12–21 × 6–10(–12) µm; basal laminal cells hyaline, firm-walled, not porose, variable in length, mostly oblong or elongate-oblong and 30–60 µm, grading gradually into the upper laminal cells; alar cells inflated, pigmented, forming a large but non-auriculate group, usually obscured by adherent rhizoids; marginal cells more elongate in <5 rows, forming a weak border confined to the base of the leaf. Costa c. (315–)350–400 µm wide and c. 40–50% of the leaf base width, clearly defined at mid leaf, excurrent or merging with hair-point, lacking lateral spurs, usually with rhizoids arising from the lower abaxial surface, in both abaxial and adaxial surface views the cells linear and thick-walled; in cross-section (mid leaf) with a median layer of guide cells, completely lacking hyalocysts ("Palinocrapsis" in anatomy), and c. 8 (–12) cell layers thick, with 3–5 layers of adaxial stereids and 2–3 layers of abaxial stereids, which are interspersed with larger cells (± the same diameter as the guide cells), the abaxial surface crenulate or with very low and single-celled ridges; in basal cross-section (just above the upper limit of the alar cells), the exposed adaxial cells small and incrassate. Propagula usually present, terminal and often clustered, c. 3 mm long and strongly distichous, consisting of modified shoots with c. 8–10 closely imbricate leaves, which are strongly cucullate and scabrid at apex.
Dioicous. Perichaetial leaves strongly sheathing, lanceolate from an oblong base, c. 5–6 mm. Perigonia terminal, often overtopped by innovations and then appearing lateral, sometimes mixed with propagula. Setae strongly cygneous, c. 8 mm; capsules ovoid, strumose or not (best seen when dry), curved and sulcate when dry, ± erect and smooth when moist, c. 1.5 mm; stomata absent; annulus well differentiated, revoluble; operculum rostrate from a conic base. Peristome teeth orange and vertically striolate below, hyaline above, split c. ⅔ to base. Calyptra not seen. Spores 15–18 µm, finely papillose.
Bartlett & Frahm 1983, fig. 1 (as C. arboricola); Malcolm & Malcolm 2006, pp. 88, 197, 222, 286.
The "Palinocrapsis" nature of the costal mid leaf cross-section, with median guide cells surrounded by abaxial and adaxial stereid bands and lacking hyalocysts, distinguishes C. purpureocaulis from all but C. clavatus in the N.Z. flora. These two species can usually be distinguished by their substrate (C. purpureocaulis predominantly lignicolous; C. clavatus predominantly on soil or rock), by the form of their propagulae (C. purpureocaulis strongly distichous; C. clavatus stellate) and by the nature of their upper laminal cells (C. purpureocaulis small and irregular, ± subquadrate and some oblate, not porose; C. clavatus mostly elongate-rhombic or rounded-rectangular, variably but often strongly porose). If capsules are present, the ovoid, sulcate capsules with peristome teeth spilt c. 2/3 to their base in C. purpureocaulis contrast with the subcylindric, non-sulcate capsules with peristome teeth that are split nearly to their base in C. clavatus.
NI: N Auckland including offshore islands (LB, GB), S Auckland (Moehau), Gisborne (Manuoha Trail, Lake Waikaremoana, Hautapu River), Hawke’s Bay (Tarawera), Taranaki (Mt Taranaki), Wellington (Mt Hauhungatahi, Ruahine Range, Tararua Range); SI: Nelson, Marlborough (Ōkaramio Saddle), Canterbury (near summit of Arthur’s Pass), Westland (Ōtira, Lake Ellery), Otago (Haast Pass), Southland; St; A; C.
Austral. Chile*, Marion I.* Also reported from Tasmania, mainland Australia (N.S.W.) and Juan Fernandez Is by Frahm (1994, p. 323).
On rotten wood, logs, horizontal branches, and on tree trunks; at higher elevations (as in the Paparoa Range of Nelson L.D.) and on Auckland and Campbell Is this species occurs on humus over rock outcrops. Such occurrences mirror the behaviour of many predominantly lignicolous species at the extremity (or maximum elevation) of their ranges. On the North I. from 720 m (Lake Waikaremoana, Gisborne L.D.) to 1150 m (Manuoha Trail, Gisborne L.D.) and on the South I. from 20 (Jackson Bay, Westland L.D.) to 1300 m (Round Lake, Nelson L.D.) elevation.
According to Frahm (1994, p. 323), C. purpureocaulis occurs, outside of Australasia, primarily in "swampy heathlands" and occurs on rotten wood only in N.Z. and Tasmania. He suggested that this unusual habitat (for a Campylopus) may be related to occurrence in hyper moist habitats.
This species is characterised in the field by its lignicolous substrate and by having distichous propagula bearing two rows of imbricate, bluntly cucullate and scabrid leaves. The propagula are borne apically and are often clustered, sometimes mixed with perigonia or perichaetia, and usually overtopped by lateral innovations. The dense brick-red rhizoids, which envelop the lower stems, and the bright yellow-green coloration of the upper, mostly hair-pointed, leaves are characteristic in a N.Z. context. It grows most frequently on decaying wood but also occurs as an epiphyte on a wide range of forest trees, including species of southern beech. The epiphytic and lignicolous habitat facilitates recognition in N.Z. Microscopically, the upper laminal cells are compact and ± subquadrate; this feature, as noted by Bartlett & Frahm (1983), assists in distinguishing the present species from C. clavatus, particularly in the absence of propagulae. The nature of the costal cross-section, with stereids on both abaxial and adaxial surfaces, is also highly characteristic.
The synonymy of the N.Z. name C. arboricola Cardot & Dixon with the Fuegian C. purpureocaulis Dusén is accepted here on the authority of Frahm (1985b; 1985a, p. 158; 1994, p. 323). Probable type material of C. purpureocaulis has been examined, but no type material of C. arboricola (with syntypes from Te Aroha, S Auckland L.D.) has been seen. The two syntypes of the latter were collected by Leland & Chase and D. Petrie, and neither collection is represented in any N.Z. herbarium.