- ≡ Bryum bartramioides Hook., Musci Exot. 1, pl. 18 (1818)
- = Rhizogonium helmsii Müll.Hal., Hedwigia 36: 333 (1897)
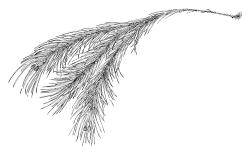
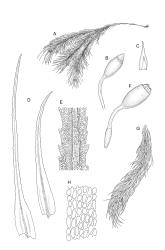
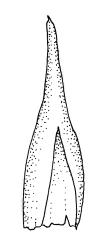
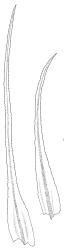
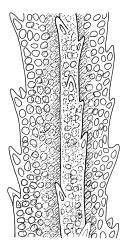
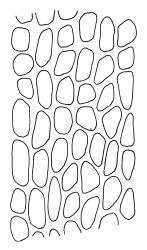
Plants very robust, loosely pendent, forming loose tufts or extensive turves, on tree-ferns, bark, or rock, yellow- or red-brown or brown-green, dull, often appearing ± dendroid. Stems very long, 50–200(–250 mm) mm, mostly unbranched below and with many, often short branches near apex, loosely pendent, curved or flexuose, with very sparse brown papillose rhizoids nearly restricted to base, densely foliose above, dichotomously branched or less often unbranched above, stiff and wiry, in cross-section with a distinct central strand and angular in outline. Leaves in many rows, densely inserted and somewhat more crowded near stem apex, weakly flexuose-secund when dry, erect-spreading and ± straight or weakly secund when moist, narrowly lanceolate (but often appearing weakly ovate at base), carinate nearly throughout, unistratose except for margins and scattered bistratose streaks, doubly toothed, c. 7–12 × 0.75–0.9 mm, those at stem base much smaller, ± ovate-lanceolate, and closely appressed; margins bistratose and much thickened above (the thickening apparent under the hand-lens), closely and doubly serrate nearly throughout, reflexed on one or both sides below; upper laminal cells irregular in shape, but many ± quadrate, thick-walled, smooth, 1–1.5:1 and 10–15 µm in greater dimension, becoming longer and more rectangular and strongly porose near leaf base; basal cells very incrassate and orange-brown in several rows; alar cells not differentiated. Costa strong, c. 105–120 µm at mid leaf, percurrent or sometimes excurrent, protruding strongly and doubly toothed on abaxial surface, in cross-section (at mid leaf) triangular, with median guide cells and large abaxial and adaxial stereid bands, the cells on the adaxial surface rectangular and mostly 2–4:1.
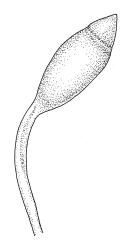
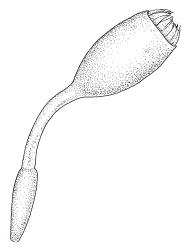
Dioicous. Perichaetia terminal, but often overtopped by subperichaetial innovation, the leaves scarcely differentiated, the archegonia numerous and mixed with filiform paraphyses. Perigonia gemmiform, c. 1.2 mm diam. (excluding outer bracts), often produced serially on one branch and subtended by innovations, the inner bracts mostly broadly ovate, concave, and pigmented, surrounding numerous antheridia and filiform paraphyses. Setae often aggregated, 1–3(–4) per perichaetium, 1–2 mm (excluding ocrea), stout, pale brown; capsules immersed, erect, broadly elliptic and ± symmetric, smooth, c. 2 mm, pale brown at maturity; mouth transverse; exothecial cells mostly oblong-hexagonal, firm-walled; stomata not seen; annulus strongly differentiated, apparently persistent at rim when operculum falls; operculum bluntly conic. Peristome double; exostome teeth yellow-brown, lanceolate and weakly shouldered, c. 650 µm, with a zig-zag median dorsal line, bordered, transversely striate below, becoming ± baculate above, with numerous ventral lamellae; endostome with a high basal membrane and segments nearly the height of the teeth, with 2–3 nodose cilia. Calyptra cucullate, smooth, and naked. Spores 12–15 µm, nearly smooth.
Brotherus 1924, fig. 381; Sainsbury 1955, pl. 44, fig. 1.
Cryptopodium bartramioides is sometimes confused with Cyrtopus setosus, which is also a robust and epiphytic plant. However, C. bartramioides is generally a larger plant with longer and narrower (7–12 × c. 0.75 mm vs c. 5–7 × 1.2–1.4 mm) and more evenly tapered and linear-lanceolate leaves. The marginal teeth in C. bartramioides are paired and readily seen with a hand-lens while those in Cyrtopus setosus are single and difficult to observe under a hand-lens. In Cryptopodium the cells of the leaf base are uniformly short-rectangular and smooth, while in Cyrtopus the laminal cells of the leaf base are dimorphic and have cuticular striations (observable under the compound microscope). The two genera also differ by several sporophytic features and by their host preferences; Cryptopodium bartramioides grows mostly on tree ferns, while Cyrtopus setosus favours smooth-barked trees, especially tawa (Beilschmiedia tawa).
Cryptopodium bartramioides could possibly be confused with Echinodium hispidum but that species is primarily terrestrial, normally dark green in colour, and has shorter, entire leaves that are subulate from an ovate or triangular base and with a long subula completely filled by the costa.
The aggregated and short setae and the elongate and strongly porose basal laminal cells are among the features that distinguish C. bartramioides from members of Pyrrhobryum. The leaves of C. bartramioides are larger (7–12 × 0.75–0.9 mm) and not as strongly reduced in size on the lower stems as are those of P. paramattense (4.5–6.0 × 0.3 mm and much reduced below). Cryptopodium bartramioides is also a more robust plant with stems nearly always in excess of 50 mm.
NI: N Auckland including offshore islands (LB), S Auckland, Gisborne, Hawke’s Bay, Taranaki, Wellington; SI: Nelson, Marlborough (Mt Stokes, Grants Pass, Pelorus Bridge Scenic Reserve, Bryant Range), Westland, Otago, Southland; St.
Endemic.
Most often occurring on tree ferns caudices and stumps (especially Cyathea smithii and C. dealbata, occasionally on Dicksonia squarrosa) where it forms dense and usually pure turves that sometimes completely obscure the fern caudex. It is also very common on rotten tree stumps in a wide range of forest types and occurs on the bark of angiosperm trees (including Elaeocarpus dentatus, Lophozonia menziesii, and Metrosideros umbellata) and probably on some podocarps, on thin humus over rock faces (including conglomerate, gneiss, schist, granite, and sandstone), and steep clay banks. This endemic species is most frequent in N Auckland and the West Coast of South I. Eastern North I. records are largely confined to the Lake Waikaremoana region; Hawke’s Bay records are entirely pre-1900. The species is rare in Marlborough and has not been collected in Canterbury (but its eventual discovery near the Main Divide seems likely).
When epiphytic, C. bartramioides has few bryophyte associates, apart from trailing hepatics (mostly Bazzania spp., and Lejeuneaceae) growing among the stems. Occurring on North I. from c. 125 m to at least 800 m (Lake Waikaremoana, Gisborne L.D.) in the east and to c. 1070 m (Mt Taranaki, Taranaki L.D.) in the west; on South I. occurring from near sea level (Pororari River, Nelson L.D.) to at least 1230 m (Scarlett Range, Nelson L.D.). The upper elevation occurrences are usually terrestrial rather than epiphytic.
The species is morphologically quite uniform throughout its N.Z. range and differs primarily in stature.
Dixon (1926, p. 223) rejected an early record of C. bartramioides from Hawai‘i by Gaudichaud, suggesting that the mounted leaves he saw were from "Rhizogonium" spiniforme; Dixon’s conclusions were for the most part accepted by subsequent workers on Hawai‘ian mosses (e.g., Bartram 1933), thus reinforcing Sainsbury’s (1955) conclusions that it is both a N.Z. endemic genus and species.