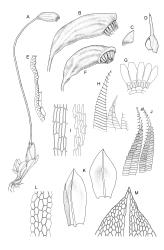
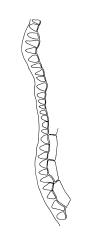
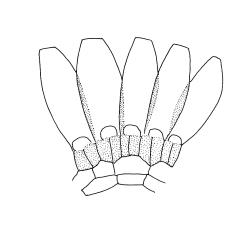
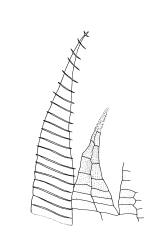
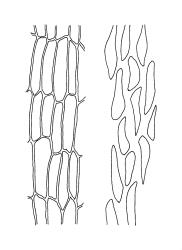
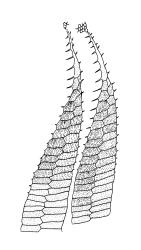
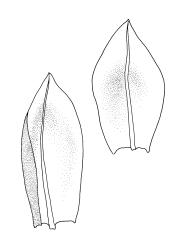
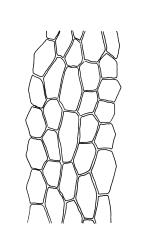
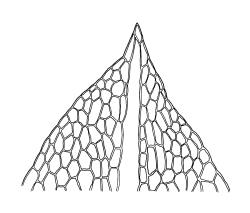
Plants medium-sized, comose, often with sporophytes large relative to the gametophytes, gregarious, usually yellow-green. Stems as per genus, with one or sometimes more innovative branches, in cross-section as per genus. Leaves erect, both moist and dry, strongly comose and clasping the base of the sporophyte, strongly concave, oblong-ovate or obovate, tapered to an acute, sometimes mucronate, apex, entire, mostly 2.0–3.8 × c. 1.6 (difficult to flatten); upper laminal cells oblong-hexagonal, lax, and smooth, mostly 36–70(–90) × 30–36 µm, but some usually smaller and ± irregular, becoming smaller near apex, and larger and more oblong near base; marginal cells not differentiated; alar cells not or scarcely differentiated. Costa red- or yellow-brown, percurrent or short-excurrent to form a mucro, c. 45–60 µm wide in lower leaf, in cross-section with a conspicuous central stereid group. Axillary hairs few, with cylindric terminal cells.
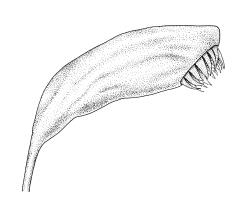
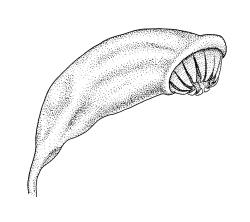
Autoicous. Perigonia and perichaetia as per genus. Setae red- or pale orange-brown at maturity, highly variable in length (to at least 50 mm), dextrorse below, sinistrorse above, smooth, strongly hygroscopic, nearly straight when dry, strongly sinuose when moist; capsules strongly asymmetric, strongly inclined when moist, semi-erect or inclined when dry, broadly ovoid-pyriform, sulcate both moist and dry, yellow-brown and becoming red-brown at maturity, with an ill-defined neck c. ⅓ to ½ the total capsule length, mostly c. 2.5–3 × c. 1.5 mm (at maturity, prior to dehiscence), with spore sac c. ½ the total capsule length; mouth oblique; exothecial cells, stomata, and annulus as per genus; operculum plano-convex, otherwise as per genus. Peristome double; exostome teeth as per genus; endostome segments from ¾ to equal the height of the teeth, pale, acuminate at apex, papillose-striolate, otherwise as per genus. Calyptra as per genus. Spores slightly subreniform, c. 10–30(–38) μm, appearing nearly smooth under the light microscope, lacking trilete scars.
Brotherus 1924, fig. 282; Flowers 1973, pl. 62, 1–8; Crum & Anderson 1981, fig. 216, A–F; Beever et al. 1992, fig. 37; Malcolm & Malcolm 2003, p. 30; Meagher & Fuhrer 2003, p. 155; Malcolm & Malcolm 2006, numerous illustrations.
There is no species in the N.Z. flora that could be confused with F. hygrometrica when fruiting. Even when non-fruiting, the large and lax laminal cells distinguish it from other taxa, except other members of Funariaceae. Sterile plants of Physcomitrium pyriforme can be difficult to distinguish from the present species.
K; NI: N Auckland including offshore islands (PK, HC, LB, RT), S Auckland, Gisborne, Hawke’s Bay, Taranaki, Wellington; SI: Nelson, Marlborough, Canterbury, Westland, Otago, Southland; St; Ch; A; C. Reported from M by Seppelt (2004).
Cosmopolitan. Tasmania*, mainland Australia* (W.A.*, S.A.*, N.S.W.* A.C.T.*, Vic.* Qld* and N.T.*). This species is virtually ubiquitous in temperate regions worldwide.
This is an extremely weedy species and listing all its potential habitats is difficult. It occurs most abundantly on mineral soil after fire. However, it occurs in a wide range of terrestrial habitats with a wide range of disturbance levels. Frequent habitats include soil heated by fire, ash piles, margins of roadside and trackside drains (with or without some saline influence), earth/clay soil banks, agricultural soils, mortar, paving stones or bricks, and nutrient-enriched soil in bird colonies. In can occupy areas of many square metres and it tolerates a wide range of soil textures. It can even rarely occur as an epiphyte, but then it is almost always associated with bird perches. On the North I. it ranges from near sea level to 2135 m (Mt Ruapehu, Wellington L.D.); on the South I. it ranges from sea level to at least 1085 m (Crown Range, Otago L.D.). Ceratodon purpureus and a range of weedy Bryum spp. are frequent associates in N.Z.
Funaria hygrometrica is a serious greenhouse weed (like Marchantia polymorpha and Leptobryum pyriforme). It has been observed growing, if not actually thriving, on commercial potting soil inside a translucent sealed plastic bag. It often survives on disturbed soil where herbicides have been applied.
Large colonies of F. hygrometrica can often be recognised from a distance by the distinctive orange-brown colour of its capsules. Fully mature capsules that have shed their spores also have a distinctive dark red-brown colour that can likewise be recognised at distance.
Much has been written about the nutrient requirements of F. hygrometrica. Hoffman (1966) found in the western U.S. that elevated levels of nitrogen and phosphorous and circum-neutral pH were the most significant factors promoting F. hygrometrica invasion of burn sites. He found that calcium, magnesium, and potassium concentrations had little influence on its growth. Southorn (1977) found that elevated ammonium concentration immediately after bonfires could be toxic to F. hygrometrica protonema, and that some leaching is required before successful colonisation can occur. He found high levels of calcium promoted growth (seemingly conflicting with the results of Hoffman), and also that F. hygrometrica might be tolerant of levels of "specific nutrients such as manganese" that are toxic to angiosperms. Such tolerances, an abbreviated life cycle, and high nutrient requirements help to explain the domination of early stages of post-fire succession by F. hygrometrica, both in N.Z. and in other parts of its range. In natural conditions in N.Z. F. hygrometrica normally completes its life cycle in less than a year, with plants observed in winter months frequently in the "spear" stage of sporophyte development.
Funaria hygrometrica also tolerates saline soils. It often occurs (often as only a small number of plants) on soil among seaside rocks subject to some salt spray. It has been collected from silt at the margin on an ephemeral inland saline lake (Salt Lake, near Sutton, Otago L.D.) by J. Child (CHR 644305). The species exhibits a wide variation in stature. Natural populations from various parts of its worldwide range have been recorded as having various ploidy levels, with n =14, 28, and 56 (Crum & Anderson 1981) and possibly other numbers; n =28 was recorded from three Australian populations (Ramsay in Löve 1967). The possibility of chromosome races being present in N.Z. has not been explored, nor has the distinct possibility that both indigenous and adventive races occur here.
This is one of the most widely recognised mosses, in part because of it weedy nature, and because it is often used as an example of a "typical" moss in introductory botany textbooks, where it is often used to illustrate a representative moss life cycle. In English it is often given the common name "Cord Moss", which alludes to the strongly twisted (like a cord or rope) setae. Another widely used common name is "Cinderella Moss", which alludes to its occurrence on ashes or cinders. This is also one of very few N.Z. mosses with a Māori name: wairua (Beever 1991).
Material from tropical parts of the range is usually referred to F. hygrometrica var. calvescens (Schwägr.) Mont., distinguished by its very long setae and longer and narrower capsules. No N.Z. collections, including the few seen from the Kermadec Is, are referable to this variety, and it is not considered further here.