- ≡ Orthotrichum luteum Mitt. in Wilson, Bot. Antarct. Voy. III. (Fl. Tasman.) Part II, 184 (1859)
- = Orthotrichum erectum R.Br.bis, Trans. & Proc. New Zealand Inst. 27: 441 (1895)
- = Orthotrichum gracillimum R.Br.bis, Trans. & Proc. New Zealand Inst. 27: 427 (1895)
- = Orthotrichum parvulum R.Br.bis, Trans. & Proc. New Zealand Inst. 27: 439 (1895)
- = Orthotrichum tortulosum R.Br.bis, Trans. & Proc. New Zealand Inst. 27: 432 (1895)
- = Orthotrichum otiraense R.Br.bis, Trans. & Proc. New Zealand Inst. 35: 333 (1903)
- = Ulota weymouthii Venturi in Rodway, Pap. & Proc. Roy. Soc. Tasmania 1912: 114 (1913)
- = Ulota bellii Malta, Acta Horti Bot. Univ. Latv. 7: 15 (1933)
- ≡ Rehubryum bellii F.Lara, Garilleti & Draper, Front. Plant Sci. 13: 10 (2022)
- = Ulota breviseta Malta, Acta Horti Bot. Univ. Latv. 7: 9 (1933)
- = Ulota laticiliata Malta, Acta Horti Bot. Univ. Latv. 7: 11 (1933)
- = Ulota bellii var. longicolla Malta, Acta Horti Bot. Univ. Latv. 7: 16 (1933)
Ulota pygmaeothecia sensu Sainsbury 1955, p. 224
Ulota rufula sensu Sainsbury 1955, p. 224
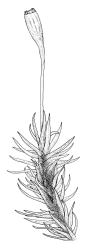
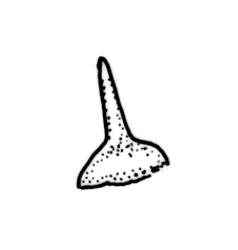
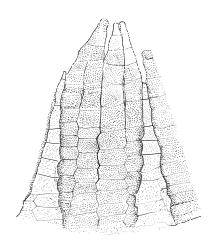
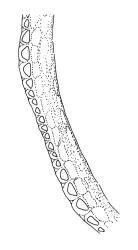
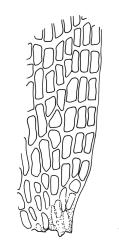
Plants yellow- to brown-green above, dark brown below. Stems sparsely branched by forking. Leaves erect-spreading when moist, strongly contorted when dry, lanceolate from a strongly defined, ovate or oblong and pigmented base, strongly constricted and reflexed at mid leaf margins, mostly crenulate, c. 1.0–2.5 × 0.4–0.5 mm (under cover slip); upper laminal cells rounded, ± isodiametric, short-elliptic, usually some oblate, mostly 9–12 μm in greater diam., unistratose, mammillose (usually with a single mammilla over the lumen); basal interior cells apparently smooth, yellow to orange, radiating from costa; cells of basal margins pale, with strongly thickened transverse walls, forming a distinct border 4–12 (rarely fewer) cells wide and extending nearly to the top of the leaf base. Costa ending shortly below the leaf apex. Gemmae absent.
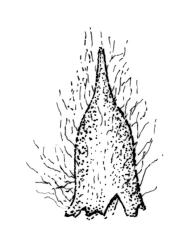
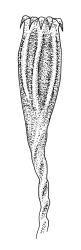
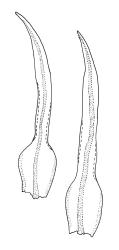
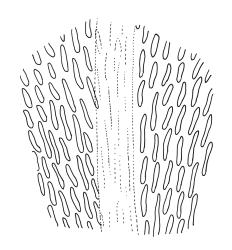
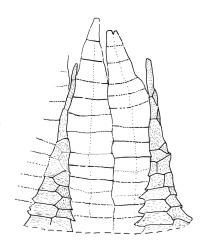
Gonioautoicous. Perichaetial leaves not differentiated. Setae elongate or rarely short, strongly dextrose; capsules exserted or rarely merely emergent, cylindric, strongly 8-ribbed when dry, c. 1.4–1.7 mm; exothecial cells as per genus; stomata restricted to neck; operculum rostrate from a conic base. Peristome double, pale; exostome teeth paired, reflexed when dry, broadly triangular, with a median zig-zag line clearly visible on both surfaces, inserted close to capsule mouth, papillose-striolate; endostome segments 8, c. 0.6–0.8 the height of the teeth, >1 cell wide and variable in width, a median line present and well-developed or ± absent, often variable in a single capsule. Calyptra campanulate, often becoming split on 1 side with age, densely hairy, enclosing the operculum and the urn of the developing capsule. Spores unicellular, spherical, thick-walled, incrassate, (30–)36–42 μm, papillose.
Malta 1933, figs 1 a–b, d–e; 2–3; Meagher & Fuhrer 2003, p. 175; Malcolm & Malcolm 2003, p. 70.
Ulota lutea appears to differ from the widespread northern hemisphere species U. crispa by having consistently larger spores and generally longer setae. However, the relationship between these species could be beneficially examined in a wider context, perhaps using non-morphological characters.
Confusion in the field can occur between the present species and both U. membranata and U. viridis. Distinguishing features are discussed under those species.
Confusion occasionally occurs between U. lutea and Orthotrichum tasmanicum var. tasmanicum. Ulota lutea has more strongly contorted leaves when dry, a more defined oblong leaf base and several marginal rows of ± colourless cells with strongly thickened transverse walls at the leaf base. The stomata in U. lutea are restricted to the base of the capsule, rather than occurring in the middle or upper half.
NI: S Auckland, Gisborne, Hawke’s Bay (Makaretu South), Taranaki, Wellington; SI: Nelson, Marlborough (Mt Stokes), Canterbury, Westland, Otago, Southland; St.
Australasian. Tasmania*. Scott & Stone (1976) recorded it from mainland Australia (Victoria).
Most commonly on southern beech (Lophozonia menziesii and Fuscospora solandri s.l.), Hoheria, and divaricating species of Coprosma, but also on many other woody genera including Aristotelia, Discaria, Dracophyllum, Fuchsia, Griselinia, Kunzea, Leptospermum, and Olearia. Also on exotic trees and shrubs including Betula, Carmichaelia, Quercus, Pinus, Salix, and Sambucus, and rarely on rock. Mostly occurring on twigs and small branches. Frequently associated with Calyptopogon mnioides, Dicnemon spp., Leptostomum inclinans, Ulota perichaetialis, and U. viridis. Ranging from 240 m (Wainuiomata Valley, Wellington L.D.) to at least 1250 m (Mt Taranaki, Taranaki L.D.) on the North I. and from c. 50 (near Berwick, Otago L.D.) to c. 1300 m (Avalanche Peak, Canterbury L.D.) on the South I.
Ulota lutea is both the most common and by far the most variable species of the genus in N.Z. The strongly contorted dry leaves, the cylindric and strongly ribbed capsules, and the lack of differentiated perichaetial leaves normally provide sufficient means of recognition for this widespread species.
Malta (1933) presented an excessively narrow concept of U. lutea. He stated in the introduction of his survey that this species could be separated from its congeners on the basis of leaf form alone. This is an oversimplification, and almost certainly the result of examination of too few specimens. The abruptness of the narrowing above the leaf base and the degree of marginal recurvature are variable in U. lutea, and these features are inadequate to permit the differentiation of the segregate species recognised by Malta.
Examination of endostomes from a range of N.Z. specimens shows segment development varies markedly in populations, and even in a single capsule (e.g., Hodgson M84, CHR 556094). In all but very few U. lutea specimens a median line is present for part of the length of some segments in each capsule. Malta’s (1933, p. 4) separation of U. laticiliata from U. lutea based on the relative width and degree of development of a median line in the endostome segments is rejected here.
Sainsbury (1955, p. 220) noted the occurrence of rudimentary "intermediate" endostome segments in some material of U. lutea. Such occurrences (i.e., an endostome with 16 segments) appear to be very rare; the only plants seen with "intermediate segments" are in the holotype of U. bellii from Lake Harris, Southland L.D. However, this feature varies even in the type of U. bellii and I could locate only eight segments in one of the two capsules examined closely. Malta himself (in herb.) considered the type of U. bellii (in H-Brotherus) to include plants of U. lutea. Ulota bellii is not worthy of taxonomic recognition.
In all material of U. lutea examined, the peristome teeth are inserted close to the capsule rim and the modest variability of this feature is insufficient to allow the drawing of taxonomic distinctions. Setae length also varies considerably across a range of specimens. These are the features emphasised by Malta (1933, p. 9) in his protologue of U. breviseta, which is here also considered a synonym of U. lutea.
Orthotrichum luteum Mitt. in Hook.f. & Wilson (the basionym of the present species) is based on two syntypes. A portion of one of the syntypes, annotated by Mitten, is present in HO. Material of the other syntype has not been seen. A lectotype should be designated in the Mitten herbarium at NY. The HO type material (presumably an isosyntype) has a small number of older capsules in which endostome detail cannot be observed. The exserted capsules are borne on rather short (c. 3 mm) setae.
Only one of the several syntypes of U. laticiliata cited by Malta (1933, p. 11) appears to be represented in the Brotherus herbarium, from which Malta borrowed the bulk of the specimens used for his revision of Australasian Ulota (see p. 3). This Tasmanian specimen (W.A. Weymouth 2487), conforms with Malta’s description and is also the material upon which he based his peristome drawings (Malta 1933, fig. 5). It is therefore selected as lectotype. Paratypes of U. laticiliata from Mt Manuoha (Gisborne L.D.) and Dawson Falls (Taranaki L.D.) are present in CHR and WELT.
The single specimen from an unspecified Marlborough locality (J. McMahon, Jan. 1936, WELT M021487!, CHR 576219!) referred to the South American Ulota rufula (Mitt.) A.Jaeger by Dixon (Sainsbury 1955, p. 225) is considered here to be aberrant U. lutea. It has capsules emergent or short-exserted beyond the perichaetial leaves rather than exserted on a long seta as in more representative material of U. lutea. In terms of both its gametophytic and sporophytic morphology, it is indistinguishable from material of so-called U. breviseta. The McMahon collection thus falls in the range of continuous variability of U. lutea in N.Z.
Sainsbury (1955, p. 224) recorded the southern South American species U. pygmaeothecia (Müll.Hal.) Besch. from Mt Taranaki. Three duplicates of Sainsbury’s collection (G.O.K. Sainsbury 777) have been examined but no well-developed sporophytes are present. The overall habit of the sterile plants is representative of U. lutea. The vegetative leaves of some shoots have fewer rows of basal marginal cells than is usual for U. lutea, but on other shoots the basal marginal cells form 5–6 rows of hyaline and thick-walled cells. Although spores (reportedly 20–24 µm diam.) have not been seen, the weight of available evidence does not support the recognition of U. pygmaeothecia as part of the N.Z. flora.
Type material (which was not examined for this study) of five species described by Robert Brown in Orthotrichum between 1895 and 1903 (in Transactions & Proceedings N.Z. Institute, vols. 27 & 35) were placed by Dixon (1926, p. 180) in the synonymy of Ulota lutea. These species are O. erectum R.Br.bis, O. gracillimum R.Br.bis, O. parvulum R.Br.bis, O. tortulosum R.Br.bis, and O. otiraense R.Br.bis. No purpose would be served by questioning these placements.