- ≡ Hypnum leucocytus Müll.Hal., Syn. Musc. Frond. 2, 314 (1851)
- ≡ Rhaphidostegium leucocytus (Müll.Hal.) A.Jaeger, Ber. Thätigk. St. Gallischen Naturwiss. Ges. 1876–1877: 403 (1878)
- ≡ Rhaphidorrhynchium leucocytus (Müll.Hal.) Broth., Nat. Pflanzenfam., ed. 2 [Engler & Prantl] 11, 427 (1925)
- ≡ Sematophyllum leucocytus (Müll.Hal.) Sainsbury, Bull. Roy. Soc. New Zealand 5: 463 (1955)
- = Hypnum cerviculatum Hook.f. & Wilson in Wilson, Bot. Antarct. Voy. II (Fl. Nov.-Zel.) Part II, 113 (1854)
- ≡ Rhaphidorrhynchium cerviculatum (Hook.f. & Wilson) M.Fleisch., Musci Buitenzorg 1249 (1923)
- = Sematophyllum macrosporum Dixon & Sainsbury in Sainsbury, Trans. & Proc. Roy. Soc. New Zealand 75: 183 (1945)
- ≡ Warburgiella macrospora (Dixon & Sainsbury) B.C.Tan, Schofield, & H.P.Ramsay, Nova Hedwigia 67: 222 (1998)
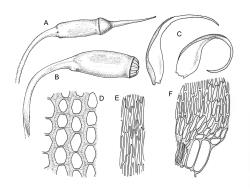
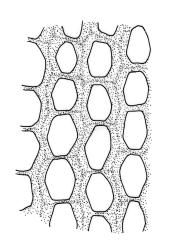
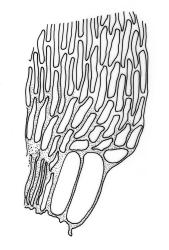
Plants lustrous, forming very fine interwoven mats. Stems irregularly branched, to at least 25 mm, in cross-section with a poorly-developed hyaloderm, several layers of incrassate outer cells and no central strand. Branches variable in length, c. 4–15 mm. Leaves strongly falcate-secund, slenderly acuminate from an ovate-lanceolate base, not or weakly twisted, strongly concave and clasping below, ± flat in the apex, plane at margins, sharply denticulate above, entire below, 1.3–1.8 × 0.3–0.35 mm (under cover slip); mid laminal cells (upper third of leaf) linear-vermicular, c. 45–60 × 3–5 µm, firm-walled, rounded and weakly prorulate, not or scarcely porose; basal cells shorter, and strongly porose and yellow; alar cells abruptly differentiated, (2–)3 strongly inflated but firm-walled, oblong-rounded, hyaline or yellow, with a few subquadrate or oblong supra-alar cells.
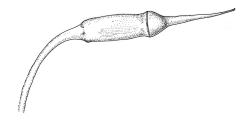
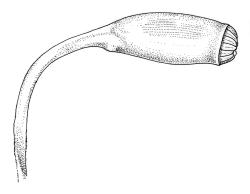
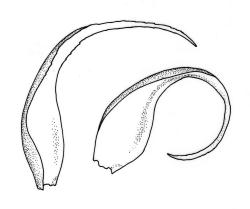
Reportedly autoicous. Perichaetia scattered on stems, the inner perichaetial leaves strongly piliferous from a ± oblong base, strongly toothed above, with alar cells not or scarcely differentiated, sheathing the seta and apparently expanding after fertilisation. Perigonia not seen. Setae red-brown, mammillate throughout, twisted weakly to the left above, (6–)9–16(–24) mm; capsules pendulous due to curvature of upper setae, oblong-cylindric, symmetric, weakly narrowed below mouth and with an irregularly protuberant-mammillate and short neck when dry, little narrowed below mouth but otherwise unchanged when moist, 1.5–2.0 mm; stomata few in neck, each situated at the apex of a protuberance and apparently immersed; operculum with a conic base and a very long, straight rostrum, c. 1.5 mm, with cells in ranks (as per exothecial cells); exothecial cells short round-oblong, arranged in distinct longitudinal ranks (visible under hand-lens), with the longitudinal walls much thicker than the transverse walls, not thickened in corners. Peristome as per genus; cilia single, nodose. Calyptra not laciniate at base. Spores (10–)15–25(–30) µm, variable in size in a single capsule, occasionally persisting as tetrads, green, smooth.
Buck et al. 2002, p. 16; Ramsay et al. 2004, fig. 24.
The striped appearance of the capsules (due to the thickened longitudinal walls of the exothecial cells) is distinctive and readily observed with a hand-lens. This feature, and the irregularly protuberant capsule neck (that Scott & Stone 1976, p. 443, described as an "angular ridge round the very base like a collapsed apophysis") make the sporophytes of this species distinctive. These are the most striking features of a species that, when sterile, is nondescript and easily confused. The low mammillae on the setae (less obvious than the papillae in some species of Brachytheciaceae) provide a further point of recognition, but these can be inconspicuous and are best observed under a compound microscope.
Some of the gametophytic recognition features mentioned by Scott & Stone (1976), including the distinction between the set of the stem and branch leaves and the whitish tinge of the plants, do not seem useful in N.Z.
Warburgiella leucocyta can easily be confounded with the much commoner Rhaphidorrhynchium amoenum, and not all sterile material can be confidently named. The nature of the alar cells may facilitate recognition in some instances. In W. leucocyta the strongly inflated extreme alar cells are firm-walled while in R. amoenum the extreme alar cells are thinner-walled (but similarly inflated). If perichaetial leaves are present, their degree of sheathing (see above under the generic discussion), abruptly tapered shape and the strongly toothed margins in W. leucocyta permit confident separation of the two taxa.
NI: N Auckland (Waitakere Ranges), S Auckland (Moehau), Taranaki (Dawson Falls), Wellington (Lake Rotopounamu, Mt Tongariro, Tararua Range, George Creek near Wainuiomata; SI: Canterbury (Maruia Springs), Westland (Kelly Range), Otago (Lake McKerrow, Dunedin, Table Hill), Southland (The Chasm, Resolution I.); St; A, C.
Austral. Mainland Australia (Victoria)*. Reported from widely scattered localities in Tasmania and eastern Australia by both Scott & Stone (1976, p. 444, as Sematophyllum leucocytus and by Ramsay et al. (2004). The latter authors also record it from South America.
On decaying wood and on tree bases in southern beech-, kamahi-, and podocarp-dominated forests. On North I. occurring from 220 m (George Creek) to 975 m (Mt Tongariro) and on South I. from sea level (Resolution I.) to c. 600 m (Maruia Springs). Frequently associated bryophytes include Catagonium politum, Cladomnion ericoides, Dicranoloma robustum, Distichophyllum pulchellum, Hypnum chrysogaster, Wijkia extenuata, and the hepatics Bazzania cf. adnexa, Chiloscyphus leucophyllus, Heteroscyphus billardierei, and Metzgeria hamata.
The sexuality of W. leucocyta cannot be recorded with confidence. I have tried repeatedly but without success to observe perigonia. Ramsay et al. (2004) recorded it as autoicous, while Scott & Stone (1976) recorded it as dioicous.
The concept of W. leucocyta presented here is broader than that of Ramsay et al. (2004). There is considerable variation in respect to spore diameter in N.Z. material (at least up to 25 µm in G.O.K. Sainsbury 4738 from Dawson Falls (CHR 606453A) and in J.E. Beever 37-02 from Mt Tongariro, CHR 406694) and with a few exceptionally large spores (up to c. 30 µm) in the holotype of S. macrosporum Dixon & Sainsbury (BM 85046). Many of the collections named as W. macrospora in N.Z. herbaria are depauperate. The distinction made by Ramsay and co-authors between W. leucocyta and W. macrospora ignores the continuous range variation of spore size. The perichaetial leaves in the type of S. macrosporum also compare well to those of representative N.Z. S. leucocytus. It is difficult to apply the differentia cited by Ramsay et al. (2004, p. 55). In N.Z. the two species cannot be differentiated.