- ≡ Hypnum amoenum Hedw., Sp. Musc. Frond. 292 (1801)
- ≡ Sematophyllum amoenum (Hedw.) Mitt., J. Linn. Soc., Bot. 12: 487 (1869)
- ≡ Rhaphidostegium amoenum (Hedw.) A.Jaeger, Ber. Thätigk. St. Gallischen Naturwiss. Ges. 1876–1877: 400 (1878)
- = Hypnum leptorhynchum Hook.f. & Wilson in Wilson, Bot. Antarct. Voy. II (Fl. Nov.-Zel.) Part II, 112 (1854)
- = Hypnum mundulum Hook.f. & Wilson in Wilson, Bot. Antarct. Voy. II (Fl. Nov.-Zel.) Part II, 112 (1854)
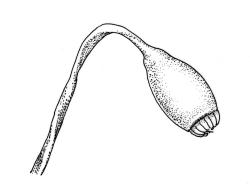
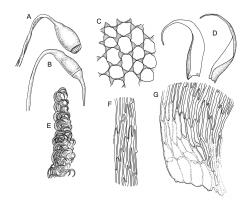
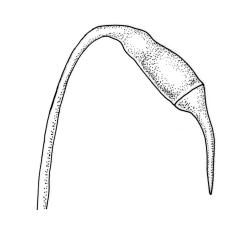
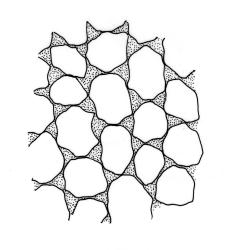
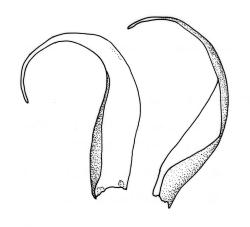
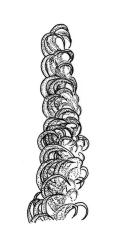
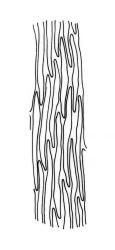
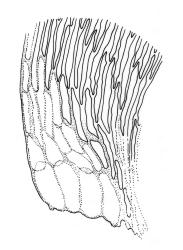
Plants pale green to yellow-green, occasionally ± bronze, moderately lustrous, forming very fine and interwoven mats. Stems subpinnately or irregularly branched, commonly c. 10–35 mm, in cross-section with 2–3 layers of incrassate outer cells and no central strand; hyaloderm apparently lacking. Branches ± uniform on a single stem, mostly c. 3–8 mm. Leaves strongly falcate-secund to nearly circinate, lanceolate from an ovate or oblong base, channelled above, narrowed to a slender and acuminate apex, entire or finely denticulate near apex, c. 1.3–1.7 × 0.20–0.30 mm (under cover slip; length difficult to measure); mid laminal cells (upper third of leaf) linear-vermicular, c. 60–90 × 3–5 µm, firm-walled, smooth, pointed at ends, not or scarcely porose; those at base shorter, yellow, and weakly to moderately porose; alar cells abruptly differentiated, 2–4 in extreme angles strongly inflated, thin-walled, oblong, and hyaline to weakly pigmented, with a few supra-alar cells subquadrate or oblong and firmer-walled. Costa lacking.
Autoicous. Perichaetia scattered on stems, the inner perichaetial leaves lanceolate and finely acuminate, c. 1.3–1.4 mm, sharply denticulate above, not distinctly sheathing, and apparently not expanding after fertilisation. Perigonia gemmiform, scattered on stem, yellow, <0.4 mm. Setae red-brown, smooth, slender, twisted weakly to the left above, 8–20 mm; capsules pendulous due to curvature of upper setae, oblong-cylindric, symmetric, narrowed below mouth when dry, neck weakly differentiated, c. 1.3–1.5 mm; stomata restricted to neck, immersed; operculum with a conic base and a very long, straight rostrum, c. 1.0 mm; exothecial cells at mid urn not in strong rows, oblong or nearly isodiametric, strongly collenchymatous, longitudinal walls and transverse walls not differentiated. Peristome double, pale; exostome teeth 16, strongly shouldered and bordered, the outer surface cross-striolate below and with a median zig-zag line, the inner surface trabeculate; endostome with a high basal membrane and mostly keeled and perforate segments nearly as long as the teeth; cilia single, nodose. Calyptra cucullate, smooth, not laciniate at base. Spores (10–)12–16(–18) µm, green, smooth.
Mueller 1864, pl. 14 (as Hypnum callidioides); Malcolm & Malcolm 2003, p. 58; Ramsay et al. 2004, fig. 17.
Ramsay et al. (2004, p. 38) noted that R. amoenum "has the appearance of a small and slender Hypnum." Rhaphidorrhynchium amoenum is often confused with Hypnum chrysogaster, a species that occupies much the same range of habitats. However R. amoenum is a finer plant, with notably shorter stem leaves (0.20–0.30 mm vs 0.5–0.7 mm in H. chrysogaster), that give the plants a different habit and usually permit separation at a glance. The supra-alar cells in R. amoenum are fewer and never oblate, as they frequently are in H. chrysogaster. The basal laminal cells adjacent to the alar cells are moderately or weakly porose in R. amoenum while those in H. chrysogaster are highly porose. Rhaphidorrhynchium amoenum is autoicous (and hence usually fruiting) while H. chrysogaster is dioicous and fruits much less frequently. When fruiting the long-rostrate operculum and collenchymatous exothecial cells of R. amoenum readily differentiate it.
Confusion occurs between R. amoenum and Warburgiella leucocyta. In R. amoenum the extreme alar cells are inflated and thin-walled whereas the extreme alar cells of W. leucocyta are inflated but firm-walled. When fertile, the smooth setae and lanceolate-acuminate and denticulate perichaetial leaves, which are not distinctly sheathing, contrast with the mammillate setae and the oblong-acuminate, coarsely serrulate and distinctly sheathing perichaetial leaves in W. leucocyta.
Some collections (especially from northern parts of the North I.) of Sematophyllum subhumile var. contiguum with leaves markedly secund can be difficult to distinguish from R. amoenum. The paler colouration of S. subhumile var. contiguum is helpful in such instances. The evenly tapered leaves with entire margins in S. subhumile var. contiguum contrast with the more channelled, more abruptly narrowed, and ± denticulate upper leaf margins in R. amoenum. If fruiting, the short setae (7–9 mm) of S. subhumile var. contiguum contrast with the usually longer (8–20 mm) and more slender setae of R. amoenum.
Sematophyllum subcylindricum is discussed under excluded taxa. Many of the collections named as S. subcylindricum in N.Z. herbaria are depauperate or aberrant R. amoenum.
K; NI: N Auckland, including offshore islands (PK, HC, LB, GB, RT), S Auckland, Gisborne, Hawke’s Bay, Taranaki, Wellington (including KA); SI: Nelson, Marlborough, Canterbury, Westland, Otago, Southland; St; Ch; A; C.
Australasian. Tasmania*, mainland Australia (N.S.W.*). Recorded from all states in Australia (excluding the Northern Territory) by Ramsay et al. (2004).
Occurring on a broad range of substrates, but best developed on rotten logs. This species of wide ecological tolerance commonly occurs on standing tree trunks, horizontal branches, and exposed roots of a broad range of native and introduced tree species, as well as on twigs (e.g., on Lophozonia menziesii, Pseudowintera colorata) and occasionally as an epiphyll (e.g., on leaves of Pseudowintera colorata). It also occurs terrestrially on humic soil, marble, and granite. Host tree species include Agathis australis, Beilschmiedia tawa, Carpodetus serratus, Coprosma spp., Dracophyllum arboreum, Fuscospora fusca, F. solandri s.l., Hoheria spp., Kunzea ericoides, Lophozonia menziesii, Melicytus ramiflorus, Metrosideros excelsa, Podocarpus laetus, and Weinmannia racemosa, and at least some tree ferns. Associated moss taxa are correspondingly numerous, but Pyrrhobryum bifarium and Wijkia extenuata s.l. are the most frequent. Other frequent moss associates include Dicranoloma billardierei, D. menziesii, D. robustum, Distichophyllum pulchellum, D. rotundifolium, numerous species of Macromitrium and Orthotrichum, Weymouthia cochlearifolia, and W. mollis, as well as a wide range of hepatics. On North I. R. amoenum ranges from near sea level to c. 1200 m (Mt Ruapehu, Wellington L.D.) and on South I. to at least 1025 m (at Mt Arthur, Nelson L.D., and Craigieburn Range, Canterbury L.D.).
This is a widespread, very common, and highly variable species. In its usual expression it has characteristic delicate, narrow, and strongly falcate leaves. It is commonly fruiting, with slender, smooth setae, pendulous capsules with obviously collenchymatous exothecial cells and finely rostrate opercula.
Hedwig (1801, p. 292) designated a type from "Seelandia". Ramsay et al. (2004, p. 37) cited, but did not examine a presumed type from "Fuegia". They did not further discuss the possibility of this species occurring in South America. No authentic South American material of this widespread austral species has been available for examination.
Sainsbury (1956) placed the Tasmanian Rhaphidostegium calliferum Hampe & Geheeb and the Victorian Rhaphidorrhynchium callidioides (Müll. Hal.) Broth. (basionym: Hypnum callidioides Müll. Hal.) in synonymy. Both these conclusions were alluded to earlier by Dixon 1929, p. 308, and followed by Ramsay et al. (2004). The types of these names have not been seen for this treatment, but there is no reason to doubt Sainsbury’s conclusions, which are corroborated by Mueller’s 1864 illustration of Hypnum callidioides. Neither of these names achieved currency in N.Z.
Hypnum cyparioides Brid. is commented on under "excluded taxa".