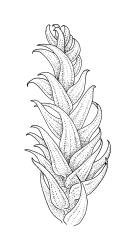
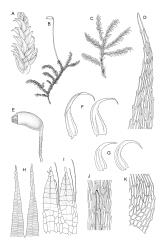
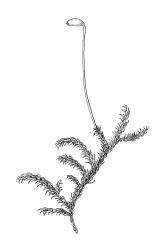
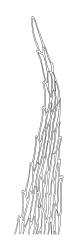
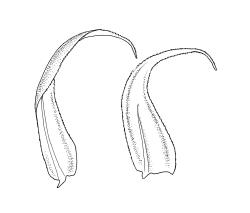
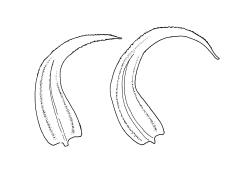
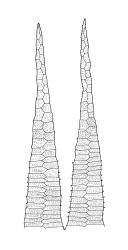
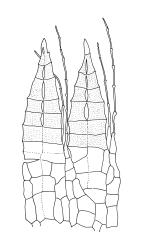
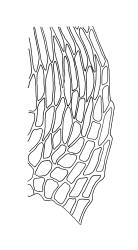
Plants medium-sized, soft to wiry, yellow- to brown-green, forming loosely to densely interwoven mats. Stems prostrate, ± pinnately branched, yellow-brown, variable in length, to at least 90 mm long, in cross-section with a central strand and 3–4 layers of thick-walled cortical cells, beset below with pale brown, smooth rhizoids. Branches variable in length, 3–17 mm long, with leaves falcate-secund. Stem leaves ± falcate-secund, symmetric, plicate, with pleats visible under the stereoscope and extending ⅔ or more the leaf length, scarcely altered when dry, ovate-lanceolate, acuminate, plane or weakly recurved below, not concave, not decurrent, denticulate (sometimes strongly or rarely weakly) above or nearly to insertion, 2.0–3.0 × c. 0.5 mm. Branch leaves smaller, 1.0–1.5(–2.0) mm long, strongly falcate-secund to nearly circinate, finely to coarsely denticulate above or to insertion, recurved at margins. Costa c. 30 µm wide (⅓ above base), extending c. ⅔ the length of the leaf, ending in a stout abaxial spine. Upper laminal cells smooth, firm-walled, linear, 60–105 × 6–8 µm; basal cells shorter in c. 3 rows; alar cells thick-walled, irregularly quadrate to short-rectangular, forming a small, poorly defined group.
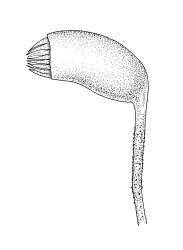
Autoicous. Perichaetia lateral on stems; perichaetial leaves acuminate from an oblong base, ecostate, widely spreading to squarrose above. Perigonia gemmiform, c. 1 mm long, scattered on stems, with ecostate, oblong-acuminate bracts and filiform (c. 7-celled) paraphyses. Setae 10–22 mm, papillose throughout, weakly sinistrorse, red-brown; capsules inclined to horizontal, asymmetric, oblong-ovoid, c. 2.0–2.8 mm long (excluding operculum), yellow- to dark brown; exothecial cells oblate to shortly rectangular. Annulus of 2–3 rows of cells; operculum bluntly conic. Exostome teeth lanceolate; endostome with well-developed, usually paired, and nodose cilia. Calyptra c. 2.5 mm long, sometimes finely pilose at base. Spores (12–)15–21 µm, finely papillose.
Brotherus 1925, fig. 688; Malcolm & Malcolm 2003, p. 6; Meagher & Fuhrer 2003, pp. 52–53.
The species most easily confused with B. paradoxum is B. velutinum, and some collections from mid elevations on the South I. are troublesome. In B. paradoxum the plicate nature of both branch and stem leaves is apparent under the stereoscope and the leaves are generally less strongly serrulate, particularly in their upper portions, and more strongly circinate than the leaves of B. velutinum. Brachythecium paradoxum is usually a golden or brown-green colour while B. velutinum is a yellow- to bright green plant. The branch leaf margins are more obviously recurved in B. paradoxum than in B. velutinum. The elevational range of the two species overlaps, but B. paradoxum is generally a higher-elevation species (c. 300 to at least 2300 m, vs near sea level to c. 700 m on the South I.).
Confusion also occurs with Sanionia uncinataof the Amblystegiaceae. The alar cells of B. paradoxum are small and thick-walled, even in the extreme alar angles, while in S. uncinata there are a few thin-walled and inflated cells in the extreme angles. A stem cross-section shows Brachythecium to lack a hyaloderm, while S. uncinata has a hyaline cortical layer, with cortical cells that often adhere to stripped-off leaves. Plants of B. paradoxum are generally less robust and have leaves that are shorter (1.0–1.5 mm vs generally ≥2.0 mm) and more strongly toothed than the leaves of S. uncinata.
Finally, B. paradoxum is sometimes confused with Austrohondaella limata. The presence of a strong single costa and papillose seta will distinguish B. paradoxum. The denticulate margins, differentiated alar cells and generally larger leaves also serve to differentiate the present species.
NI: S Auckland (Maungapōhatu, several locations near Taupō), Gisborne (Lake Waikaremoana), Hawke’s Bay (Titiokura), Taranaki (Mt Egmont), Wellington; SI: Nelson, Marlborough (Inland Kaikōura Range), Canterbury, Westland (Ōtira), Otago, Southland.
Austral. Tasmania*, mainland Australia*, southern South America*, Kerguelen*, Crozet Is*, Marion I.* Reported from southern Africa by Hedenäs (2002), although this report might be based on an earlier record from “Afr. 4” by van der Wijk et al. (1959, p. 216) that was almost certainly founded on records from Kerguelen.
This is a versatile species that can occur on soil, rock, duff, rotten wood, or rarely on bark, in a wide range of vegetation types, including Fuscospora solandri and broad-leaved forests, mānuka scrub, tussock grasslands, and alpine vegetation. It mostly avoids moist situations and rarely occurs as an epiphyte. It can grow on a variety of rock types, including greywacke, limestone, and basalt. The bulk of records are from the South I. east of the Main Divide, but the species is also particularly common on the higher-elevation limestone/marble in Nelson L.D. Known from 610 (Ōhakune, Wellington L.D.) to at least 1600 m (Wilkes Pool Gorge, Mt Egmont, Taranaki L.D.) on the North I. On the South I. ranging from c. 300 (Mt Thomas, Canterbury L.D.) to at least 2300 m (Inland Kaikōura Range, Marlborough L.D.), although collections from below 600 m are few in number. Cladomnion ericoides, Ditrichum spp., Hylocomium splendens, Leptotheca gaudichaudii, Palamocladium leskeoides, Pohlia cruda, Rosulabryum billardierei, and Tortella knightii are frequent associates.