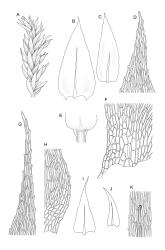
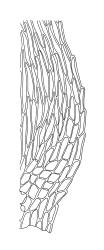
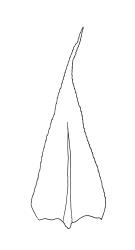
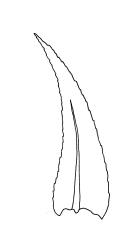
Plants small, soft, yellow- to bright green, silky, forming loosely interwoven mats. Stems prostrate, irregularly to subpinnately branched, pale green, to c. 25(–55) mm long, in cross-section with 3–4 layers of thick-walled cortical cells, beset below with pale brown, smooth rhizoids. Branches c. 3–8 mm long, with leaves falcate-secund. Stem leaves falcate-secund (especially at stem tips), symmetric, not or only weakly plicate, scarcely altered when dry, narrowly ovate-lanceolate, acuminate and ± twisted at apex, plane, scarcely concave, weakly decurrent, mostly coarsely and sharply serrulate near tip and serrulations extending to insertion or nearly so, 1.4–2.0 × 0.3–0.5 mm. Branch leaves smaller, 0.9–1.3(–1.5) × c. 0.3 mm, with a smaller alar group than stem leaves, not decurrent. Costa 24–30 µm wide (⅓ above base) extending c. ½ the length of the leaf (ending well below the base of acumen), ending in a conspicuous abaxial spine and often toothed abaxially for c. ⅓ of its length. Upper laminal cells smooth, firm-walled, linear, c. 56–80(–110) × 5–7 µm; basal cells shorter in 3–5 rows; alar cells (of stem leaves) poorly delimited, thick-walled, irregularly quadrate to short-rectangular, extending to c. 10 cells up the margin and c. ½ to the costa, not extending into the decurrency.
Autoicous. Perichaetial leaves ovate-lanceolate, ecostate, erect-spreading. Perigonia ovoid-gemmiform, scattered on branches, with ecostate, ovate, shortly acuminate bracts and filiform (c. 7‑celled) paraphyses. Setae c. 9–16 mm, papillose throughout, c. 180 µm diam., weakly sinistrorse, red-brown; capsules horizontal to pendulous, asymmetric, shortly oblong-ovoid, 1.5–2 mm long (excluding operculum), pale to dark yellowish-brown; exothecial cells ± oblong, 30–60 µm. Annulus of 2–3 rows of cells, falling with the operculum. Operculum bluntly conic. Exostome teeth lanceolate; endostome with well-developed nodose cilia in groups of 2–3. Calyptra c. 2 mm long. Spores 15–19 µm, finely papillose.
Crum & Anderson 1981, fig. 503; Smith 2004, fig. 278, 1–5.
The abaxial costal spine here is generally easily seen and is occasionally so robust as to be visible under high magnification (40–50×) of the stereoscope. The spores of N.Z. material are larger than indicated in the description in some northern hemisphere floras (e.g., Crum & Anderson 1981).
This species can be easily confused with B. paradoxum. The two species are usually separable by the smooth or weakly plicate and more strongly toothed leaves of B. velutinum. In B. paradoxum the plicate and strongly circinate nature of both the branch and stem leaves is apparent under the stereoscope. The multiple abaxial spines at the costa terminus in B. velutinum can also be helpful in difficult instances. Also, the leaf margins (especially of the branch leaves) are not recurved in B. velutinum but are clearly recurved in B. paradoxum. Brachythecium velutinum is a yellow- to bright green plant, while B. paradoxum is usually a golden or brown-green colour. The two species overlap in their elevational distributions, but B. velutinum is not known to occur above 700 m, while B. paradoxum occurs mostly at higher elevations.
SI: Canterbury, Otago, Southland (Te Ānau). Reported from C by Vitt (1974), but this record seems very unlikely, and the voucher specimen has not been seen.
Adventive. Widespread in the northern hemisphere.
On soil in dry situations, including grazed areas. Often occurring on tree bases, on vertical soil or rocky banks (including limestone), and under tussocks. Often on bases or lower trunks of willows and sometimes associated with mānuka (Leptospermum scoparium s.l.) or matagouri (Discaria toumatou). The eventual finding of this species in drier parts of the North I. seems likely. Ranging from near sea level (Kākānui, Otago L.D.) to c. 700 m (Ōtepatōtū Scenic Reserve, Canterbury L.D.). Associated moss species include Bartramia papillata, Encalypta rhaptocarpa, Hypnum cupressiforme, Hedwigidium integrifolium, Philonotis scabrifolia, Syntrichia antarctica, and Thuidiopsis furfurosa.
Only one specimen collected prior to 1945 has been confirmed; it was collected in the Christchurch Botanical Garden in 1891 (W. Bell, CHR 471556). The near absence of early collections, the association of this species with exotic plantations and/or grazed or otherwise modified native vegetation, and a lack of reports from either Tasmania or mainland Australia (Hedenäs 2002) strongly suggest that B. velutinum is adventive in N.Z. Its restricted distribution, occurrence at low elevations, and apparent absence from other southern hemisphere regions are consistent with its proposed adventive status.