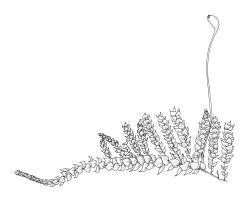
Plants delicate to robust, forming loose wefts, compact mats, or erect turves, terrestrial, epiphytic, or epilithic. Stems creeping or ascending, loosely and irregularly to densely and pinnately branched, mostly with a central strand, the branches straight or sometimes curved. Leaves mostly crowded in several rows, occasionally complanate, those of stems and branches differentiated or not, mostly erect-spreading, less often imbricate or wide-spreading, occasionally secund, ovate to lanceolate and mostly acuminate, rarely broadly ovate and obtuse or rounded at apex, plicate, striolate or smooth, usually denticulate above at margins. Laminal cells linear to elongate-hexagonal or elongate-rhomboid, smooth or occasionally prorate, mostly shorter and often porose near insertion; alar cells ± differentiated, often subquadrate, and often forming well-marked groups. Costa nearly always single, well developed but mostly ending below the leaf apex, often projecting as a terminal abaxial spine. Paraphyllia none. Pseudoparaphyllia present or not.
Sexuality various. Perichaetial leaves differentiated, sheathing or spreading. Setae elongate, often papillose by projecting cell ends; capsules inclined to horizontal, rarely erect, mostly asymmetric and relatively short, often broadly ovoid, occasionally ovoid-cylindric or cylindric, usually dark; stomata round, restricted to the capsule base; annulus differentiated or not; operculum bluntly conic to long-rostrate. Peristome double, mostly perfect; exostome teeth cross-striolate below, mostly dark yellow-brown; endostome mostly with a high basal membrane, keeled and perforate segments and well-developed nodose cilia, but sometimes cilia, segments, or membrane reduced. Calyptra cucullate, smooth or rarely sparsely hairy. Spores small, papillose to nearly smooth.
The Brachytheciaceae are a large family of cosmopolitan distribution. Hedenäs (2002, quoting Walther 1983) estimated the family to include more than 30 genera and nearly 700 species worldwide; Brotherus (1925, p. 350) recognised 23 genera. Nine genera and 21 species are recognised as part of the N.Z. flora, although two genera and several species are clearly adventive here.
There is little consensus concerning either the family limits or infra-familial generic limits. For these reasons both the family concept and the generic concepts presented here are largely traditional ones. For the most part they conform to concepts employed by Hedenäs (2002) in his treatment of Australian representatives. However, I disagree with Hedenäs in respect to the status of the genus Eriodon, which is recognised here as a distinct genus because of its highly distinct peristome. Also, the genus Palamocladium, which occurs in N.Z., is not known from Australia.
Confusion can occur between the Brachytheciaceae and the often superficially similar members of the Amblystegiaceae. Buck (1998, p. 234) presented a brief but useful discussion of features he considered distinguished these two families.
The Brachytheciaceae are placed in the order Hypnobryales (Hypnales) in 20th century classification schemes, including the influential treatments of Brotherus (1925), Vitt (1984), and Goffinet et al. (2009)
Ignatov (1999) has examined the morphogenesis of pseudoparaphyllia in members of the Brachytheciaceae and the Meteoriaceae and found that the pattern of development in these two families is “unique", and supported Buck’s (1994) earlier hypothesis that several elements traditionally placed in the Meteoriaceae are better placed within a broadly defined Brachytheciaceae. Ignatov & Huttunen (2003, p. 257) emphasised pseudoparaphyll morphogenesis in a selection of diagnostic characters for the family. According to them, the only morphological features that “consistently characterize(s) members of the Brachytheciaceae and at the same time excluding [sic] members of other families” are: the orientation and angle between the first-formed pseudoparaphyllia and the later-formed pseudoparaphyllia, the presence/absence of papillae above central laminal cell lumina, and the (round) shape of the stomatal pore.
Ignatov & Huttunen (2003) presented a phylogenetic consideration using both molecular and morphological data from the Brachytheciaceae, which largely confirms Buck’s (1994) hypothesis. They proposed a new generic and subfamilial classification of the Brachytheciaceae, which treats 41 genera within four subfamilies; but the reliability of both their generic and higher-level (e.g. subfamily) taxonomic classification requires further assessment.
The overview of the family in Australia (Hedenäs 2002) is particularly useful for assessing N.Z. material; useful regional treatments of the family for other geographic areas include those of Buck (1998), Crum & Anderson (1981), Ignatov et al. (1999), Newton (1979), and Smith (2004).
| 1 | Branches julaceous and terete; leaves imbricate, strongly concave, minutely reflexed at apex, and broadly oblong-ovate to nearly cochleariform | 2 |
| 1' | Branches not julaceous (if terete the branch leaves widely spreading); leaves neither imbricate nor minutely reflexed at apex, mostly narrower | 3 |
| 2 | Plants loosely interwoven and ascendant; stem leaves mostly 1.5–2.1 × 1.0–1.5 mm; costae ½–⅔ the leaf length, lacking a terminal spicule; branches not curved; common and weedy at lower elevations throughout, especially in roughly mown lawns, pastures, and roadside and track verges | Pseudoscleropodium |
| 2' | Plants more compact and prostrate; stem leaves smaller, 1.0–1.6 × 0.6–0.8 mm; costae ¾–⅞ the leaf length, mostly with an inconspicuous abaxial terminal spicule; branches mostly distinctly curved; poorly known and documented only from the Wellington Botanical Gardens and Havelock North | Scleropodium |
| 3 | Leaves striolate to plicate, especially when dry | 4 |
| 3' | Leaves not plicate, smooth or somewhat striolate when dry (if striolate then apical cells much shorter than mid laminal cells and plants ± aquatic) | 6 |
| 4 | Dioicous, not known to fruit in N.Z.; plants often pendent; leaves straight, narrowly lanceolate from a subcordate base, 3.0–4.0 mm long; mostly restricted to limestone and marble, occasionally epiphytic, but never aquatic | Palamocladium |
| 4' | Autoicous and frequently fruiting; plants not or rarely pendent, forming interwoven mats (if pendent, then plants aquatic); leaves broader (not narrowly lanceolate), nearly always <3.0 mm; on a range of substrates including soil, rotten wood, or bark and rock; sometimes aquatic | 5 |
| 5 | Plants aquatic; stem leaves neither differing in shape nor markedly larger than branch leaves, broadly ovate and broadly acute at apex, moderately homomallous, not falcate-secund; well-developed branch leaves mostly 1.0–1.3 mm wide | Platyhypnidium |
| 5' | Plants terrestrial or occasionally epiphytic, not aquatic; stem leaves differentiated by shape and larger than branch leaves, ovate-lanceolate, falcate-secund to nearly circinate; well-developed branch leaves <0.5 mm wide | Brachythecium pro parte (B. paradoxum) |
| 6 | Plants extremely small; stem leaves c. 0.6–0.7 mm, broadly acute or obtuse; alar cells differentiated, quadrate, forming a large group extending nearly to the costa | Scorpiurium |
| 6' | Plants larger; stem leaves when well developed >1 mm, acute or acuminate; alar cells variously differentiated but not extending nearly to the costa | 7 |
| 7 | Operculum conic or short rostrate | Brachythecium |
| 7' | Operculum rostrate or long subulate | 8 |
| 8 | Capsules narrowly cylindric; endostome membrane short, with cilia lacking or rudimentary and linear, non-perforate segments; setae smooth, long and slender; usually epiphytic | Eriodon |
| 8' | Capsules mostly broadly obovoid to short-cylindric, not narrowly cylindric; endostome membrane tall, with cilia well developed and wider, perforate segments; setae scabrous or smooth; terrestrial or epiphytic | 9 |
| 9 | Plants aquatic, robust; branch leaves usually striolate when dry, often ± secund when moist, broadly acute at apex; cells of the leaf apices markedly shorter than mid laminal cells | Platyhypnidium |
| 9' | Plants not aquatic, small, moderate or robust in size; branch leaves neither plicate nor secund, acuminate at apex; cells of the leaf apices nearly the same length as mid laminal cells | 10 |
| 10 | Stem leaves either markedly decurrent or cordate | Eurhynchium pro parte |
| 10' | Stem leaves not or weakly decurrent, not cordate | 11 |
| 11 | Branch leaves broadly ovate to nearly elliptic; costa of branch leaves stout and nearly uniform in width throughout, mostly ¾–⅞ the leaf length; spores mostly 18–21 µm; northern in distribution | Eurhynchium pro parte (E. speciosum) |
| 11' | Branch leaves ovate-acuminate, ovate-lanceolate, or broadly ovate and acuminate, not elliptic; costa of branch leaves distinctly tapered, delicate, shorter (mostly ⅔ the leaf length or less); spores smaller, mostly 12–15 µm; widespread | Rhynchostegium |
| Category | Number |
|---|---|
| Indigenous (Endemic) | 1 |
| Indigenous (Non-endemic) | 12 |
| Exotic: Fully Naturalised | 8 |
| Total | 21 |