- ≡ Hookeria tenella Hook.f. & Wilson in Wilson, Bot. Antarct. Voy. II (Fl. Nov.-Zel.) Part II, 122 (1854)
- = Sauloma macrospora Sainsbury, Rev. Bryol. Lichénol., n.s. 18: 113 (1949)
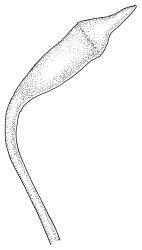
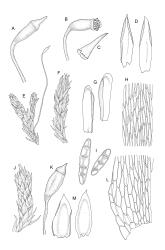
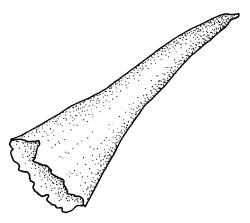
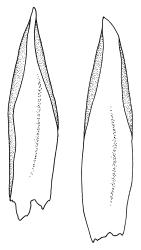
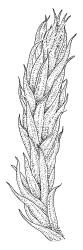
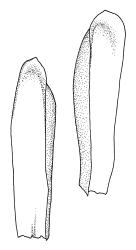
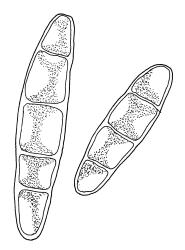
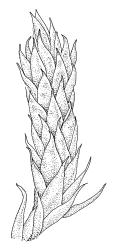
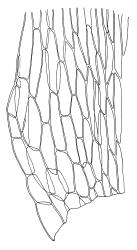
Plants medium-sized, soft, pale white-green or gold-brown, usually erect, sometimes prostrate or pendent, forming mats. Stems to 60 mm, weakly erect and self-supporting, delicate, pale, becoming yellow- or red-brown in lower portions, in cross section composed of nearly uniform parenchyma cells, with weakly differentiated cortical cells and no central strand; rhizoids restricted to basal part of stem, sparse, smooth, and pale. Shoots not complanate, c. 2 mm wide. Leaves closely inserted in several ranks, erect-spreading when moist, constricted and appearing narrower and homomallous when dry, oblong to ovate-lanceolate, rounded, broadly obtuse, acute, to weakly acuminate and mostly reflexed at apex, concave, entire or nearly so, (1.5–)1.8–2.3 × 0.6–0.9 mm. Costa absent or very weak and double, occasionally reaching to mid leaf. Upper laminal cells linear-rhomboid to fusiform, smooth, thin-walled, porose, 75–135(–165) × 12–15(–18) μm and 5–8:1, becoming longer (to c. 180 µm) in lower lamina and shorter and somewhat wider at insertion; alar cells oblong and weakly inflated to form a moderate-sized but ill-defined group. Gemmae borne on elongate filaments in leaf axils, fusiform, with 3–5 transverse walls, c. 120 μm long.
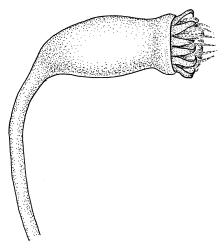
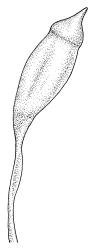
Dioicous. Perichaetia lateral or at base of stems; perichaetial leaves smaller than vegetative, oblong-ovate, c. 1.0 mm long. Perigonia terminal, with rather large, broadly ovate, strongly concave gold-brown bracts enclosing c. 20 antheridia with biseriate stalks (c. 100–120 μm long) and filiform 4–5‑celled paraphyses. Setae (4–)6–12 mm, in cross-section with a cortical layer of 2–3 layers of thick-walled cells, lacking a central strand; capsules ovoid, from an ill-defined neck, (0.8–)1.0–1.8(–2.0) mm, constricted below the mouth and warty when mature and dry, pale brown; exothecial cells mostly rounded-quadrate, strongly collenchymatous. Operculum high-conic, 0.5–1.0 mm long. Peristome teeth c. 425 μm long, otherwise as per genus; endostome with perforate segments equal to the teeth in length, appearing longer than the exostome when dry. Calyptra mitrate, c. 1.0–2.0 mm long, somewhat lacerate but not fimbriate at base, naked, very pale, covering the operculum and c. upper 1/3 of the capsule. Spores extremely variable in size, 10–48(–54) μm diam., green, nearly smooth, often germinating in capsule.
Brotherus 1925, fig. 598, F–K; Streimann 2000, fig. 19.
Sterile plants of S. tenella could be confused with Hampeella alaris, but the former is a paler plant with larger leaves lacking inrolled margins. The larger upper laminal cells (mostly 75–135 × 12–15 μm in S. tenella vs 60–90 × 4–5 μm in H. alaris), the very different alar cells, and the dimension and number of cells of the gemmae (c. 120 μm and 4–6-celled vs c. 1000 μm and 20–25-celled in H. alaris) distinguish these two species. They also differ by many sporophytic features. Streimann (2000) suggested that S. tenella could be confused with some species of Sematophyllum (possibly bleached material?), but this seems unlikely in a N.Z. context.
NI: N Auckland (Ōmahuta State Forest), S Auckland (Kaimai Range, Rotorua–Taupō region), Gisborne (Te Tiki, Ruakituri River), Hawke’s Bay (Dannevirke), Taranaki (Dawson Falls), Wellington (Mt Ruapehu, Wanganui, Mt Bruce, Carterton, Eastbourne); SI: Nelson, Marlborough (Mt Richmond State Forest, Molesworth), Canterbury, Westland (Ōtira, Franz Josef, Robinsons Creek), Otago (Hunter Valley, Pine Hill, Paradise), Southland; St (Port Pegasus).
Austral. Tasmania*, Argentina*. Reported from mainland Australia (including one locality in WA) and Chile by Streimann (2000).
Extremely catholic in regard to substrate, S. tenella grows on stumps/logs and on rock (including limestone, sandstone, scoria, and greywacke) in damp situations. It is often in seeps and occurs rarely on sheltered earth banks and as an epiphyte. As an epiphyte it can occur on trunks, branches, and small twigs, the last mostly in hyper-moist subalpine situations. Fuscospora solandri s.l. is the most frequently recorded host plant, but S. tenella also occurs on other genera, including Hoheria, Cyathodes, Coprosma, Fuchsia, and Dacrycarpus. It is primarily a forest species, but extends above the tree-line in protected situations. Sauloma tenella appears to be a rare species north of c. 37° 30' S (the approximate latitude of Huntly, S Auckland L.D.). Occurring from low elevations to at least c. 1200 m (Dawson Falls) and probably to c. 1800 m on the North I., and from 40 (Bullock Creek, Nelson L.D.) to c. 1500 m (Culliford Hill, Nelson L.D.) on the South I.
Sauloma tenella is a highly variable species with respect to both gametophytic and sporophytic characters. The plants are most commonly self-supporting and mat-forming. The mats can assume a horizontal or even pendent stance, especially on the sides of logs or in seeps. The nature of the leaf apices, ranging from rounded to acute and from erect to strongly reflexed, varies markedly even within a single population. Collections occur in which stems with reflexed leaf apices and erect leaf apices are present in roughly equal numbers (e.g., K.W. Allison 7164 from Lewis Pass, CHR 491641; G.B. Huang 451 from Broad Stream, Canterbury L.D., CHR 462976). Some collections have both reflexed and obtuse leaf apices and erect and acute leaf apices (e.g., G. Brownlie 137 from Arthur’s Pass, CHR 427989, and A.J. Fife 6754 from Cass, Canterbury L.D., CHR 405820). The type collection of S. macrospora provides an example of a population in which leaf apices are predominantly erect and acute, but with a few leaves with reflexed apices. Populations also occur in which the majority of stems have acute leaves with weakly reflexed apices. The variation of the vegetative leaves seems to be continuous and, although strongly reflexed leaf apices seem to be more common on the South I., a similar range of variability occurs throughout the two main islands. The type of S. tenella has erect, acute to weakly acuminate leaf apices and recurved upper leaf margins; it lacks mature capsules.
In addition to gametophytic characters, capsule length and spore dimension vary greatly. Sainsbury (1949; 1955) suggested that larger capsules (i.e., those greater than 1.5 mm) are correlated with subacute or obtuse, reflexed leaf apices. My observations do not support this contention. Material from Temple Basin in Arthur’s Pass (e.g., A.J. Fife 7373, CHR 406583) with predominantly reflexed and obtuse leaf apices has capsules 0.8–1.0 mm. These capsule lengths correspond to the dimensions that Sainsbury (1955) accepted for S. tenella. Material in which all leaves are acute to acuminate, but in which some capsules greatly exceed the dimensions described by Sainsbury (1955) for S. tenella, also occurs (e.g., K.W. Allison 3255 from Rangitāiki, S Auckland L.D., CHR 466226). Even the type of S. macrospora poses problems using the leaf and capsule characters utilised by Sainsbury to separate the two alleged species of Sauloma. In the type, capsules range in length from 0.8 to 1.8 mm and the majority of stems have erect leaf apices, but some leaves are markedly reflexed.
Spore dimensions vary more than suggested by Sainsbury (1955), who gave spore diameters of 12–14 µm and 20–32 µm for S. tenella and S. macrospora, respectively. Only a minority of herbarium specimens have mature spores. Spore dimensions range continuously from c. 10 to 54 µm, with extreme variability even within single populations. Spore dimensions do not appear to correlate with gametophytic characters. In two specimens (A.J. Fife 8696 from Cobb Valley, Nelson L.D., CHR 460812, and J.E. Beever 36-05a from Mt Ruapehu, CHR 406712) with extremely large spores (30–48 µm in both cases), a large percentage have germinated within the dehiscent capsules, and protonemal filaments have emerged. Small numbers of germinated spores have also been observed in other collections. This suggests that spore dimension variability may be a function of maturity/germination state and casts further doubt upon the taxonomic value of S. macrospora.
Sainsbury (1955) suggested that axillary gemmae occur exclusively or primarily in populations with larger spores and reflexed leaf apices. In my experience the presence of gemmae does not correlate with spore size or leaf apex reflexion. K.W. Allison 3255 from Rangitāiki is an example of a collection with both erect, acute leaf apices and axillary gemmae. Likewise the distinctions that Sainsbury drew using endostome segment perforations and laminal cell dimensions cannot be supported.
Sauloma presents a bewildering degree of variation. However, none of the characters proposed by Sainsbury (1955) for segregating S. macrospora from S. tenella stand up to scrutiny. Interestingly, the holotype of S. macrospora bears the following notation by Dixon (in Sainsbury’s hand): "I feel doubtful therefore whether it [the type of S. macrospora] is more than a form or variety of tenella… I have seen no intermediate forms except Brotherus’ figure! But I hesitate to call it a n. sp." My own observations support Dixon’s opinion, and it is best to take a broad view of S. tenella.