- = Macromitrium rigescens Broth. & Dixon in Dixon, J. Linn. Soc., Bot. 40: 446 (1912)
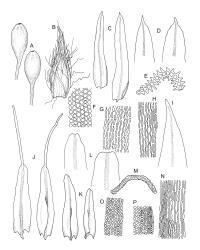
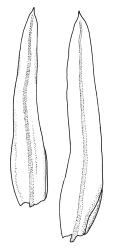
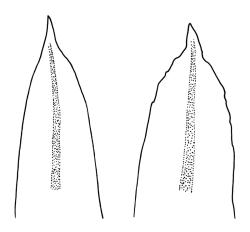
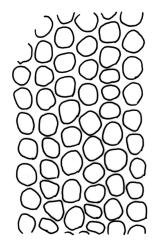
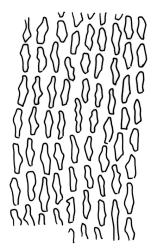
Plants robust but with short branches, dull, yellow- to olive-green above, rust- or chocolate-brown below, mostly on bark. Branches short, to 10 mm, ascendant, simple or forking below perichaetia. Stem leaves erect-flexuose when dry, wide-spreading-recurved when moist, broadly lanceolate, gradually acuminate, 1.6–2.0 mm. Branch leaves neither funiculate nor spiralled around the branch, strongly and irregularly twisted when dry, often twisting 360° or more in upper portions, spreading and ± flexuose when moist, ovate- or lanceolate-ligulate, abruptly acute-cuspidate or broadly acuminate, keeled and usually with a single plication below, 2.5–4.5 mm; margins broadly reflexed on one margin below (obvious when dry), subentire to crenulate, sometimes with irregular but shallow indentations near apex; upper laminal cells isodiametric and rounded, firm-walled and slightly thickened at corners, strongly bulging, both surfaces with one strong central papilla, unistratose, 10–16 μm diam. (lumina c. 9–12 μm diam.); papillae tall (to c. 15–18 μm high) and usually rounded distally, simple or irregular or weakly branched at their apices; mid laminal cells similar to upper or more elliptic, to c. 18 μm long, extending basally with little change in shape very far toward the leaf base (usually to within c. 500–800 μm of the leaf insertion) and there transitioning rapidly into the inner basal cells; inner basal cells elongate, with thick and ± pitted walls, golden, irregularly linear, smooth or tuberculate, c. (15–)20–40 μm long, with lumina mostly 3–5 µm wide; marginal basal cells not differentiated. Costa ending a few cells below apex, with abaxial superficial cells elongate, but obscured by short and papillose cells in upper portion, in mid leaf cross-section with 2–3 rows of abaxial stereids and 3–4 adaxial guide cells. Gemmae absent.
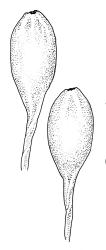
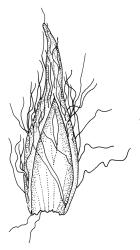
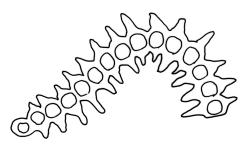
Pseudautoicous. Perichaetial leaves slightly smaller and more ovate than branch leaves, not sheathing, surrounding a densely hairy vaginula. Male plants dwarf and epiphytic. Setae 2.5–4.5 mm, smooth, erect or flexuose, strongly dextrorse; capsules oblong-elliptic, smooth below, strongly narrowed and 8-plicate at mouth, the plicae sometimes extending downwards to the upper ⅓ of the capsule with age, erect, dark-brown and ± shiny, 1.5–2.0 mm; exothecial cells oblong or irregularly elliptic, thick-walled, mostly 20–60 μm long, shorter, rounded and thicker walled near rim; stomata sparse; annulus and operculum as per genus. Peristome absent. Calyptra large, c. 3.0–3.5 mm (excluding the apical hairs), splitting by 1–3 deep slits, smooth, covered with dense, long, and smooth yellow hairs. Spores anisosporous, 18–40(–50) μm, finely papillose, smaller spores thin-walled, larger spores moderately thick-walled.
Vitt 1983, figs 123–133, 219–220, 222–223, 226.
Macromitrium grossirete is frequently confused with M. prorepens but the latter species has branched papillae, 4-plicate capsules, a peristome, excurrent costae and lacerate, hairy calyptrae. Papillose upper leaf cells are also found in M. ligulare, which is best differentiated by very small, simple papillae on the bulging upper leaf cells and naked calyptrae. These two species have sinistrorse setae, in contrast to the dextrose setae of M. grossirete. Other species with dextrorse setae include M. longirostre and M. retusum, which, however, have ± smooth upper laminal cells.
NI: N Auckland (near Auckland), S Auckland (near Tauranga, near Rangitāiki), Wellington (Hauhungatahi, Kiwi Mouth Hut, Ruahine Range, near Wellington); SI: Nelson, Marlborough (Richmond Range, Black Birch Range, near Kēkerengū), Canterbury (including Banks Peninsula), Westland, Otago, Southland (Lake Manapōuri, Lake Hauroko); Ch.
Endemic.
Macromitrium grossirete is poorly documented and apparently uncommon on the North I.; Vitt (1983) records it from five widely scattered localities and specifically confirms its occurrence in gallery forest near the tree line on Mt Ruapehu. The only North I. fertile collection I have seen is from near Kiwi Mouth Hut in the Kaweka Range (L. Perrie & L. Shepherd s.n., 11 Dec. 2003, WELT M036174). I have seen little material from Marlborough and none from coastal areas of Otago, but it is recorded from these localities by Vitt (1983). This is the commonest Macromitrium in the montane southern beech forest east of the Main Divide in Canterbury.
Occurring primarily on trunks and larger branches of Fuscospora cliffortioides, F. solandri, and Lophozonia menziesii. Infrequent host species include Coprosma parviflora, Dracophyllum uniflorum, Griselinia littoralis, Kunzea ericoides, Leptospermum scoparium, Lophomyrtus obcordata, Pittosporum tenuifolium, Phyllocladus alpinus, Podocarpus laetus, and Populus sp. This species also occasionally occurs on rock. Frequently associated epiphytes include Calyptopogon mnioides, Dicnemon semicryptum, Hypnum cupressiforme var. filiforme, Leptostomum inclinans, Macrocoma tenue, and Orthotrichum tasmanicum. It ranges in elevation from near sea level (Chaneys, Canterbury L.D.) to 1200 m (Nelson Lakes National Park, Nelson L.D.) on the South I., with the majority of collections from above c. 400 m in montane regions.
Macromitrium grossirete is a very distinctive species characterised gametophytically by robust but shortly branched plants, long and irregularly twisted leaves, strongly singly papillose upper laminal cells, an abrupt transition between the mid laminal cells and the elongate inner basal cells (which extend a relatively short distance above the leaf base), a percurrent costa, and a hairy vaginula. Sporophytically, gymnostomous capsules that are strongly narrowed and 8-plicate at the mouth, dextrorse setae, and calyptrae with only two or three or sometimes one major slit, and slender, dense, non-papillose hairs provide further distinction. I agree with Vitt (1983) that the extremely large mid leaf papillae can be seen with a stereoscope in this species, and this further facilitates recognition.
Despite the frequent production of capsules in this species, I have not located male plants in any herbarium material, despite targeted searching (especially in CHR 462846 and 592170, both of which are amply fruiting) and must accept Vitt's (1983) observations that this species has dwarf male plants.
Vitt & Ramsay (1985) included M. grossirete in their "M. hemitrichodes group" along with M. prorepens, M. submucronifolium (which is not recognised here), M. angulatum (excluded here from the N.Z. flora), and three Australian endemics.