- ≡ Orthotrichum prorepens Hook., Musci Exot. 2, 120 (1819)
- = Macromitrium submucronifolium Müll.Hal. & Hampe, Linnaea 26: 499 (1855)
- = Macromitrium erosulum Mitt., J. Proc. Linn. Soc., Bot. 4: 78 (1859)
- = Macromitrium coarctatulum Müll.Hal., Hedwigia 37: 153 (1898)
- = Macromitrium oocarpum Müll.Hal., Hedwigia 37: 157 (1898)
- = Macromitrium papillifolium Müll.Hal., Hedwigia 37: 154 (1898)
- = Macromitrium petriei Dixon, Bull. Torrey Bot. Club 42: 101 (1915)
- = Macromitrium prorepens var. aristata Allison, Trans. Roy. Soc. New Zealand 88: 10 (1960)
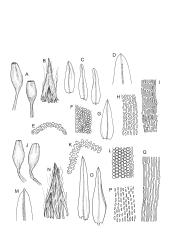
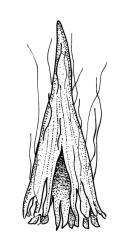
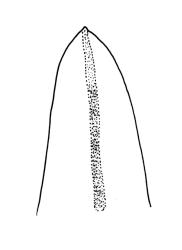
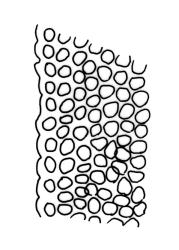
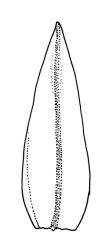
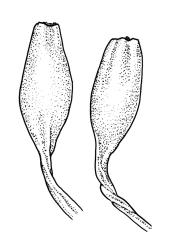
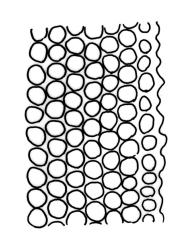
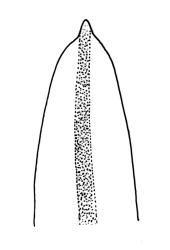
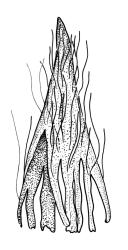
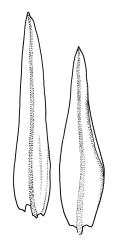
Plants slender or robust, dull, yellow- to dark olive-green above, darker or brown below, on bark. Branches variable in length, 3–20 mm, ascendant, simple or forking below sex organs. Stem leaves erect-appressed when dry, widely spreading-recurved when moist, ovate-lanceolate, 1.0–1.5 mm. Branch leaves irregularly twisted-flexuose to weakly twisted around the branch and often with apices decurved when dry, stiffly spreading or erect-spreading and not funiculate when moist, ovate-oblong, oblong-lanceolate, or lingulate and rounded, obtuse or broadly acute at apex, with a short, stout apiculus, keeled, often with a single plica below, 1.2–2.5(–3.0) mm; margins entire or weakly crenulate, recurved on one or both sides below; upper laminal cells mostly rounded-quadrate, thick-walled, usually obscure in surface view, ± bulging, unistratose, densely (1–)2–4 papillose (papillae often forked), (6–)8–16 µm wide; mid laminal cells similar in shape and size or elliptic and longer, mostly unipapillose, in distinct longitudinal rows, the transition to the basal cells gradual; inner basal cells elongate-rectangular, with ± uniform and thick cell walls (lumina 2–4 µm wide), smooth or with a few high and conical papillae, yellow, usually extending ¼ to ⅓ the leaf length, 20–45(–60) µm; basal marginal cells sometimes thinner-walled and wider than adjacent cells, forming a short indistinct border. Costa short-excurrent and filling the apiculus or rarely percurrent, ± straight (not curving to one side above), the abaxial superficial cells elongate except at apex where obscured by quadrate cells, in cross-section with 1–3 rows of abaxial stereids and 2–4 guide cells. Gemmae absent.
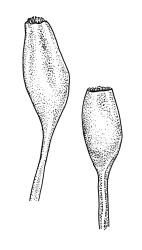
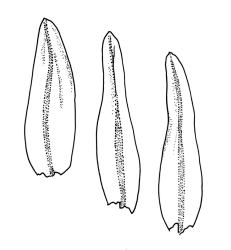
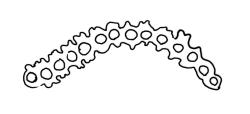
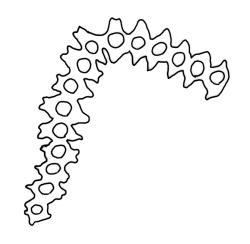
Pseudautoicous. Perichaetial leaves not differentiated, surrounding a sparsely hairy vaginula. Dwarf males axillary, bulbiform, c. 0.5 mm, the bracts broadly ovate. Setae variable in length, 2–9 mm, smooth, sinistrorse, flexuose or erect; capsules oblong-ovoid, smooth below, gradually narrowed from the middle and 4-plicate and dark beneath the mouth, (0.8–)1.0–1.8 mm; exothecial cells irregularly elliptic to rectangular, ± thick-walled, mostly 25–70 µm, shorter, darker, and with much-thickened longitudinal walls near mouth; stomata and annulus as per genus; operculum as per genus, very long, c. 1.0 mm. Peristome single; exostome teeth 16, well-developed, pale, erect, ± flexuose, 130–150 µm, papillose on outer surface, papillose from transverse striae on inner surface. Calyptra evenly and deeply lacerate, strongly plicate, sparsely or less often densely hairy with thick, yellow, ± stiff or twisted hairs. Spores anisosporous, 16–44 µm, finely papillose.
Macromitrium prorepens s.l. is sometimes confused with M. ligulare. Macromitrium prorepens has leaves twisted-flexuose to weakly twisted around the branches when dry, costae usually excurrent to form short apiculi, and usually hairy calyptrae, while M. ligulare has branch leaves irregularly flexuose-twisted when dry, subpercurrent costae, and naked calyptrae. The length and shape of the inner basal laminal cells also help to distinguish these two species. In M. prorepens s.l. the inner basal laminal cells are 20–45(–60) µm, elongate-rectangular, with ± uniform and thick cell walls, and lumina 2–4 µm wide, while in M. ligulare the inner basal laminal cells are shortly rectangular, 14–28 µm and have lumina c. 4–6 µm wide.
NI: N Auckland, including offshore islands (PK, LB, RT), S Auckland, Gisborne, Hawke’s Bay Wellington; SI: Nelson, Canterbury, Marlborough (Mataura I., Pelorus Bridge Scenic Reserve), Westland, Otago (eastern regions); Southland; St.
Endemic.
While most common on Lophozonia menziesii, this species is also epiphytic on Beilschmiedia spp., Carpodetus serratus, Coprosma spp., Fuscospora fusca, F. solandri s.l., Griselinia littoralis, Leptospermum scoparium, Leucopogon fasciculatus, Melicytus ramiflorus, Metrosideros excelsa, Pseudowintera colorata, and Weinmannia spp., as well as the gymnosperms Dacrycarpus cupressinum, Phyllocladus trichomanoides, Podocarpus totara, and Prumnopitys taxifolia. It rarely occurs on rock. It ranges from sea level to c. 1070 m (Ruahine Range, Hawke’s Bay L.D.) on the North I. and from sea level to c. 1150 m (Paparoa Range, Nelson L.D.) on the South I. It appears to occur most frequently below c. 700 m.
Frequent bryophyte associates include Cladomnion ericoides, Dichelodontium nitidulum, Dicnemon spp., Holomitrium perichaetiale, Hypnum cupressiforme var. filiforme, Leptostomum spp., Lepyrodon australis, Macrocoma tenue, Macromitrium retusum, Mesotus celatus, Neckera hymenodonta, Papillaria flavolimbata, Weymouthia spp., and the hepatics Frullania spp., Jamesoniella monodon, Plagiochila circumdentata, and P. circinalis.
Macromitrium prorepens is a highly variable and taxonomically troublesome species. It is given some character by branch leaves being usually weakly twisted around the branch and apically decurved when dry, densely papillose upper leaf cells, short excurrent or percurrent costae, sinistrorse setae, 4-plicate capsules, hairy and evenly lacerate calyptrae, and anisosporous spores.
The distinction between M. prorepens and M. submucronifolium Müll.Hal. & Hampe has been a long-standing problem of N.Z. bryology. The expedient solution of placing M. submucronifolium in synonymy with the former is adopted here. Macromitrium submucronifolium has also been closely linked to a third and widely-applied N.Z. name, M. erosulum Mitt.
Dixon (1926, p. 185) placed M. submucronifolium in the synonymy of M. prorepens while also distinguishing M. erosulum Mitt. He considered M. prorepens to be "common throughout New Zealand" and described M. erosulum as "probably one of the commonest species".
Sainsbury (1955) distinguished these two closely allied species (M. prorepens and M. erosulum), but described M. prorepens as "a weak species [which] certainly intergrades with the latter". Sainsbury described M. erosulum as "a common species and [one] very variable in habit, position of the leaves when dry, and the nature and degree of the papillosity of the leaf-cells". In his revision of Macromitrium in N.Z., Vitt (1983) recognised both M. prorepens and M. submucronifolium (with M. erosulum as a synonym of the latter) but admitted considerable difficulty in distinguishing them.
Faced with a wide range of material, I have repeatedly been unable to apply Vitt’s key dichotomy 9 (see his key on p. 7), which distinguished two taxa using the relative width of trabeculae and lumina of their medial leaf cells. Likewise, I have been unable to use the nature of the middle lamellae of the upper laminal cells to distinguish the two taxa. In all other characters employed by Vitt, including seta length, I find overlap between the two hypothetical taxa.
Due to my inability to confidently differentiate the two taxa, M. submucronifolium and M. erosulum Mitt. are here treated as taxonomic synonyms of the older M. prorepens (Hook.) Schwägr. Recognition of these taxa at an infra-specific rank would accomplish nothing and my intuition (contrary to that of Vitt) is that even a detailed morphometric study would remain inconclusive. I admit, given this broad interpretation, that M. prorepens is a highly variable and generally unsatisfactory species. However, distinguishing two species or infraspecific taxa within this range of variability requires making unjustifiable and arbitrary distinctions.
Vitt (1983) discussed the relationship between M. prorepens and M. submucronifolium at length. In a summary of his difficulty distinguishing them (p. 57) he stated: "variation in M. submucronifolium is broad enough to over-lap that in M. prorepens, with small plants of the former species hardly distinguishable from those of the latter." Vitt continued: "despite this over-lap in variation, I feel the typical expressions of these species are significantly different to warrant the recognition of these taxa as separate species. In this case, my [Vitt’s] intuitive feeling is that this structural variation does not indicate interbreeding and genetic intergradation. Rather there are two distinct species, with the variation of one variable taxon over-lapping that of a less variable taxon". However, elsewhere (p. 56) Vitt states that he is "not totally convinced that M. [sub] mucronifolium can be differentiated from M. prorepens in all cases," and that "this seeming intergradation serves to make identification very tenuous at times". Vitt considered (p. 60) M. prorepens to be a "species with limited variability" and M. submucronifolium to be "sometimes quite variable." For students attempting to separate these two taxa using Vitt’s criteria, I recommend close reading of his discussion of the problem.
Macromitrium prorepens is placed by Vitt & Ramsay (1985) in their "M. hemitrichodes group" with M. grossirete and M. submucronifolium (not recognised here). Three species (including M. hemitrichodes) endemic to Australia and the Malesian species M. angulatum are also placed in this group.