Rhizomes erect, scaly. Rhizome scales narrowly ovate, 4.5–10 mm long, 1–4 mm wide, pale brown, concolorous, entire. Fronds 380–1430 mm long. Stipes 165–800 mm long, red-brown proximally, yellow-brown or chestnut-brown distally, with scattered pale brown scales proximally, up to 15 mm long and 4.5 mm wide, and multicellular hairs amongst the scales when young. Rachises yellow-brown or chestnut-brown, narrowly winged in distal half, bearing scattered ovate scales. Laminae 2-pinnate-pinnatifid to 3-pinnate-pinnatifid, 200–800 mm long, 200–900 mm wide, ovate to broadly ovate, dark shiny green on adaxial surface, paler on the abaxial surface, coriaceous, bearing scattered ovate scales on the costae. Primary pinnae in 3–9 pairs below pinnatifid apex, overlapping; the longest at or near base, 135–520 mm long, 80–320 mm wide, ovate to broadly ovate, straight; pinna apices acute to acuminate, bases adnate on distal pinnae, stalked on proximal pinnae. Longest secondary pinnae 65–240 mm long, 17–120 mm wide, ovate to narrowly ovate, sub-opposite; apices acute to acuminate, bases usually adnate or with 1 pair short-stalked; basal 1–2 basiscopic secondary pinnae on basal primary pinnae markedly longer than others. Longest tertiary segments 9–85 mm long, 4–28 mm wide, elliptic to oblong; apices acute to obtuse, margins serrate or divided to more than halfway in largest fronds, bases adnate. Veins anastomosing. Sori elongated along margins of the ultimate segments. Mean exospore diameter 33–36 μm.
Pteris carsei is distinguished by its erect rhizome, laminae that are shiny green adaxially, and 1-pinnate-pinnatifid distally to 2-pinnate-pinnatifid or rarely 3-pinnate proximally, anastomosing veins, overlapping pinnae, broad coriaceous ultimate segments, and secondary pinnae that are mostly adnate or sessile on the lowermost primary pinnae. The species is believed to hybridise with both P. macilenta and P. saxatilis, making identification difficult in coastal areas of northern New Zealand. However, the greatly extended basal basiscopic secondary pinnae, and the mostly adnate secondary pinnae on the lower primary pinnae, distinguish P. carsei from P. macilenta and P. saxatilis. It differs from P. epaleata by its dark shiny green, rather than dull yellow-green, adaxial lamina surface, less divided lamina, and presence of ovate, rather than hair-like, scales on the rachis and pinna costae. P. carsei occurs in the northern half of the North Island, whereas P. epaleata is confined to southern Fiordland.
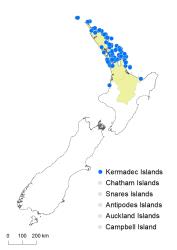
North Island: Northland, Auckland, Volcanic Plateau
Kermadec Islands, Three Kings Islands.
Altitudinal range: 0–275 m.
Pteris carsei grows on the Kermadec and Three Kings Islands, and on the Hauraki Gulf and Coromandel islands as far south as Mayor Island (Tuhua). On the mainland it is found in coastal districts along the east coast from Te Paki to Auckland. On the west coast it occurs at Spirits Bay, around Kaitāia, the northern Kaipara Harbour, and either side of the Manukau Harbour. There are outlying populations near Ōpōtiki in the eastern Bay of Plenty, and at Ngarupupu Point north of Mōkau. It grows from sea level, reaching c. 275 m near Unuwhao.
Pteris carsei is a terrestrial fern found in open coastal sites, in coastal forest under Metrosideros excelsa, Kunzea spp., and other coastal broadleaved species. It favours rocky streambeds and streamsides, rocky forest, cliffs, hillsides and beach fronts near the sea.
Pteris carsei is known to hybridise with P. saxatilis in coastal areas of Northland and Auckland, often forming extensive hybrid swarms when the two occur together (see suites of material in AK). Spores of putative hybrids are sometimes abnormal in appearance, but aborted spores are not as easy to detect as in genera such as Asplenium or Polystichum. There may be some degree of fertility in hybrids between Pteris carsei and P. saxatilis, and back-crosses to both parents could be possible. Whether P. carsei also crosses with P. macilenta is not known with certainty. The two hybrid combinations with P. carsei are almost impossible to distinguish morphologically in herbarium material, but hybrids between P. carsei and P. macilenta are likely to have aborted spores because of the difference in chromosome number. Braggins (1975) created artificial hybrids between the diploid species, P. carsei and P. saxatilis, and it is probable on morphological grounds that P. macilenta is an allotetraploid derivative of wild hybrids between the two diploid species. However, experimental evidence for this is lacking, and all three species have very similar chloroplast DNA sequences, indicating a close relationship (Bouma et al. 2010).
n = 30 (Brownlie 1961); 2n = 58 (Walker 1962).
Brownsey et al. (2020) showed that P. carsei is an endemic species distinct from P. comans and other species in the tropical Pacific. Pteris comans sens. str. occurs in the Solomon Islands, Vanuatu and Fiji and may also occur on New Caledonia, Samoa and elsewhere in the Pacific. Pteris zahlbruckneriana (syn. P. endlicheriana J.Ag.) from Norfolk Island, and P. microptera from Lord Howe Island, both belong in the P. comans complex, but are distinct from P. carsei (Braggins 1975; Green 1994; Brownsey et al. 2020). The status of plants referred to the poorly known P. laevis Mett. from New Caledonia and P. litoralis Rech.f. from Samoa and Vanuatu require further investigation.