- Asplenium ×lucrosum
- Asplenium aethiopicum
- Asplenium appendiculatum
- Asplenium bulbiferum
- Asplenium chathamense
- Asplenium cimmeriorum
- Asplenium decurrens
- Asplenium flabellifolium
- Asplenium flaccidum
- Asplenium gracillimum
- Asplenium hookerianum
- Asplenium lamprophyllum
- Asplenium lepidotum
- Asplenium lyallii
- Asplenium oblongifolium
- Asplenium obtusatum
- Asplenium pauperequitum
- Asplenium polyodon
- Asplenium richardii
- Asplenium scleroprium
- Asplenium scolopendrium
- Asplenium shuttleworthianum
- Asplenium subglandulosum
- Asplenium trichomanes
- = Phyllitis Hill, Brit. Herb. 525 (1757)
- = Caenopteris Bergius, Acta Acad. Sci. Imp. Petrop. 1782(2): 249, t. 7 (1786)
- = Darea Juss., Gen. Pl. 15 (1789)
- = Ceterach Willd., Anleit. Selbststud. Bot. 578 (1804)
- = Tarachia C.Presl, Epimel. Bot. 74 (1851)
- = Pleurosorus Fée, Mém. Foug., 5. Gen. Filic. 179, t. 16c (1852)
- = Chamaefilix Hill ex Farw., Amer. Midl. Naturalist 12: 239 (1931)
Terrestrial, rupestral or epiphytic ferns. Rhizomes erect or short- to long-creeping, bearing clathrate scales. Fronds monomorphic or rarely dimorphic. Laminae undivided or 1-pinnate to 4-pinnate-pinnatifid, membranous (not NZ) or herbaceous to coriaceous (NZ), usually bearing clathrate scales and sometimes hairs. Veins usually free or rarely reticulate (not NZ). Sori elongate on veins, superficial, borne on abaxial surface, usually away from margins but sometimes submarginal; paraphyses absent; indusia rarely absent, usually elongate with free ends or rarely very short or pouched, usually attached to one side of vein or rarely back to back on both sides. Sporangia brown or rarely orange-brown. Spores monolete, dark brown; perispores winged, cristate, echinate or reticulate, with plain, fenestrate, or reticulate areoles.
Aspleniaceae is dominated by the genus Asplenium, which includes over 700 species (PPG 1 2016). Numerous attempts have been made to subdivide the genus, with up to 20 segregates proposed, mainly on rather minor morphological characters, but only a few small groups have been widely recognised. Rather more convincing is the phylogenetic analysis of Schneider et al. (2004), who identified eight major clades based on rbcL and trnL-F spacer sequences amongst 71 species from throughout the world. This was enhanced by analysis of sequences from the same chloroplast regions for 21 taxa of indigenous New Zealand species (Perrie & Brownsey 2005a), and a further 21 taxa from Australia (Ohlsen et al. 2014). Both studies supported the clades originally identified by Schneider et al. (2004), and both showed that Asplenium was not monophyletic in either New Zealand or Australia. In fact, five of the eight clades of Schneider et al. (2004) are present in the two countries. Ohlsen et al. (2014) recognised a total of 13 clades, with some of the original eight clades further subdivided. In particular, Clade VII of Schneider et al. (2004) comprises several distinct subclades, two of which occur in New Zealand and are here considered separate clades (Clades IX and XI). The major clades in Asplenium have yet to be recognised in a formal taxonomic sense, and it is uncertain whether they correspond to any of the previously named segregates, except that most of the bird’s nest ferns fall into a clade that might correspond to Thamnopteris (C.Presl) C.Presl, while the northern hemisphere A. ruta-muraria L. together with species previously assigned to Pleurosorus occupy another. The majority of New Zealand and Australian species fall within Clade V of Schneider et al. (2004) along with, rather surprisingly, the bird’s nest ferns (Perrie & Brownsey 2005a; Ohlsen et al. 2014).
Allan (1961) accepted 12 species of Asplenium and one of Pleurosorus in New Zealand. Brownsey (1977b) revised the group and recognised 14 species and four subspecies of Asplenium. Subsequently a further four new species were described (Brownsey & Jackson 1984; Brownsey 1985; Brownsey & de Lange 1997; Perrie & Brownsey 2016), three subspecies were raised to species rank (Ogle 1988), and three introduced species or hybrids were identified (Brownsey in Webb et al. 1988; Heenan et al. 2004; Perrie et al. 2005). A total of 21 indigenous species and three introduced species or named hybrids is accepted here, almost doubling the number in Allan’s Flora.
In addition, Brownsey (1977a) recognised 19 different hybrids that occurred naturally in the New Zealand flora, a number that has since doubled to 37 combinations (see Hybrid combinations, Table 1). All produce abnormally formed or aborted spores and are thought to be effectively sterile. Hybrids between species of Asplenium account for more than half the total number of hybrid fern combinations recorded in New Zealand (Breitwieser et al. 2012), but despite these 37 different combinations, five indigenous species (A. flabellifolium, A. pauperequitum, A. polyodon, A. subglandulosum and A. trichomanes) are not known to hybridise with any other species in New Zealand. The species that do hybridise are now known to belong to Clade V of Ohlsen et al. (2014), whereas those that do not hybridise belong to Clades III, VI, IX, and XI, reinforcing the view that the eight groupings have real phylogenetic significance.
Brownsey (1977b) showed that polyploidy had been of considerable importance in the evolution of Asplenium in New Zealand, as in other parts of the world (Kramer & Viane 1990). There are no diploids in New Zealand, and all taxa are either tetraploid (11), hexaploid (2), or octoploid (10); A. trichomanes has both tetraploid and hexaploid cytotypes, and A. flabellifolium both hexaploid and octoploid cytotypes. Evidence from morphology (Brownsey 1977b, Brownsey 1977a) and DNA sequencing (Perrie & Brownsey 2005b; Shepherd et al. 2008) strongly suggests that seven octoploid species have arisen by allopolyploidy and one by autopolyploidy, in each case with at least one of the tetraploid parents still extant in New Zealand. Some have originated more than once from the same parent species (Perrie et al. 2010).
New Zealand indigenous species are assigned to the following Clades of Ohlsen et al. (2014; also see Schneider et al. 2004; Perrie & Brownsey 2005a):
Clade III: A. subglandulosum (1 species).
Clade V: A. appendiculatum, A. bulbiferum, A. chathamense, A. cimmeriorum, A. decurrens, A. flaccidum, A. gracillimum, A. hookerianum, A. lamprophyllum, A. lepidotum, A. lyallii, A. oblongifolium, A. obtusatum, A. richardii, A. scleroprium, A. shuttleworthianum (16 species).
Clade VI: A. polyodon (1 species).
Clade IX: A. flabellifolium, A. pauperequitum (2 species).
Clade XI: A. trichomanes (1 species)
| 1 | Laminae undivided | 2 |
| Laminae 1-pinnatifid or more divided | 3 | |
| 2 | Laminae usually <120 mm long; lamina bases cuneate; sori attached on one side of a vein (very rarely, plants of oblongifolium will also key here, but can be distinguished by their hair-like scales) | lepidotum |
| Laminae usually >120 mm long; lamina bases with two expanded lobes clasping the stipe; sori attached on both sides of a vein | scolopendrium | |
| 3 | Laminae densely covered in glandular or non-glandular hairs | subglandulosum |
| Laminae often scaly but never hairy | 4 | |
| 4 | Stipes and rachises dark brown throughout except for the extreme distal tip | 5 |
| Stipes and rachises green in at least the distal half | 8 | |
| 5 | Primary pinnae in 1–5 pairs, with a broad undivided apical segment | pauperequitum |
| Primary pinnae in 5 or more pairs, with a small pinnate or pinnatifid apical segment | 6 | |
| 6 | Laminae at least 2-pinnate | aethiopicum |
| Laminae 1-pinnate; pinnae often toothed but not deeply divided | 7 | |
| 7 | Laminae <20 mm wide; abaxial and adaxial surfaces of similar colour; pinna margins entire or shallowly crenate or serrate | trichomanes |
| Laminae >40 mm wide; abaxial surface paler green than adaxial surface; pinna margins doubly and often deeply serrate | polyodon | |
| 8 | Laminae 1-pinnate; primary pinna margins toothed but not divided more than halfway to the costa | 9 |
| Lamina 1-pinnate-pinnatifd or more divided; at least one pair of primary pinnae lobed or divided more than halfway to the costa | 16 | |
| 9 | Longest primary pinnae >10 times as long as broad | flaccidum subsp. flaccidum |
| Longest primary pinnae <10 times as long as broad | 10 | |
| 10 | Primary pinnae flabellate, about as long as broad; rachises extended distally and rooting at tips | flabellifolium |
| Primary pinnae oblong, narrowly elliptic or narrowly ovate, at least twice as long as broad; rachises not extended distally or rooting at tips | 11 | |
| 11 | Margins of primary pinnae prominently and regularly toothed; sori almost reaching margins at sinuses | scleroprium |
| Margins of primary pinnae entire or shallowly toothed; sori not reaching margins | 12 | |
| 12 | Scales on stipe and rachis very narrow, with long hair-like apices; pinna apices acuminate to acute or rarely obtuse; perispores reticulate | oblongifolium |
| Scales on stipe and rachis ovate or narrowly ovate, lacking long hair-like apices; pinna apices acute to obtuse or rounded; perispores winged or ridged but not reticulate | 13 | |
| 13 | Adaxial surfaces of pinnae bearing abundant scales; plants growing on base-rich soils in inland areas | 14 |
| Adaxial surfaces of pinnae lacking scales except sometimes on the costae; plants of coastal habitats growing in immediate vicinity of salt spray | 15 | |
| 14 | Terminal pinna undivided or with a proximal pinnatifid lobe, incised no more than primary pinnae; spores 37–42 μm long, 23–28 μm wide* | lepidotum |
| Terminal pinna pinnatifid, or more deeply incised than the distal margins of the primary pinnae; spores 40–54 μm long, 27–35 μm wide* | lyallii | |
| 15 | Scales on stipe and rachis narrowly ovate, with short filiform apices; laminae 23–370 (470) mm long, 15–160 (210) mm wide; spores 45–52 μm long, 29–33 μm wide*; plants growing from Kermadec Islands to north Taranaki | decurrens |
| Scales on stipe and rachis ovate, with very short filiform apices; laminae 27–540 mm long, 23–290 mm wide; spores 40–43 μm long, 24–27 μm wide*; plants growing from Kapiti Island to the subantarctic islands | obtusatum | |
| 16 | Laminae bearing bulbils on adaxial surface | 17 |
| Laminae lacking bubils | 19 | |
| 17 | Primary pinnae dimorphic, the fertile narrower and more divided than the sterile, occurring distally; spores aborted | ×lucrosum |
| Primary pinnae not dimorphic; spores normal | 18 | |
| 18 | Laminae often bearing abundant bulbils; stipe scales ovate or broadly ovate, lacking filiform apices; spores 34–37 μm long, 23–26 μm wide* | bulbiferum |
| Laminae usually bearing only scattered bulbils; stipe scales ovate or narrowly ovate, with filiform apices; spores 41–48 μm long, 28–32 μm wide* | gracillimum | |
| 19 | Rhizomes short-creeping | 20 |
| Rhizomes erect | 21 | |
| 20 | Laminae glossy green on adaxial surface, 140–590 mm long; indusia 2–10 mm long; free margins of indusia entire | lamprophyllum |
| Laminae dull green on adaxial surface, 10–78 mm long; indusia 1.5–3.5 mm long; free margins of indusia sometimes irregularly incised | cimmeriorum | |
| 21 | Basal acroscopic secondary pinna on each primary pinna much longer and more divided than the others | 22 |
| Secondary pinnae on each primary pinna all about the same size, or decreasing gradually in size and division towards the distal end | 23 | |
| 22 | Fronds firm and upright; spores 40–49 μm long, 26–33 μm wide*; plants of coastal rocks and cliffs, or low epiphytes on Metrosideros, in northern North Island and Kermadec Islands | flaccidum subsp.haurakiense |
| Fronds limp and pendulous; spores 35–46 μm long, 21–30 μm wide*; epiphytic plants of forest throughout New Zealand | flaccidum subsp. flaccidum | |
| 23 | Sori away from pinna margins | 24 |
| Sori submarginal | 27 | |
| 24 | Indusia 3–14 mm long; laminae never more than 2-pinnate | lyallii |
| Indusia 1–4 mm long; laminae often more than 2-pinnate | 25 | |
| 25 | Laminae with 3–18 pairs of pinnae; secondary pinnae with long slender stalks | hookerianum |
| Laminae with 6–40 pairs of pinnae; secondary pinnae sessile or with short, broad stalks | 26 | |
| 26 | Stipe scales ovate or broadly ovate, lacking filiform apices; spores 34–37 μm long, 23–26 μm wide* | bulbiferum |
| Stipe scales ovate or narrowly ovate with filiform apices; spores 41–48 μm long, 28–32 μm wide* | gracillimum | |
| 27 | Laminae 1-pinnate-pinnatifid | 28 |
| Laminae at least 2-pinnate | 29 | |
| 28 | Secondary segments on each primary pinna all linear and about equal in size | flaccidum subsp. flaccidum |
| Secondary segments on each primary pinna decreasing in size and/or division towards its distal end | appendiculatum | |
| 29 | Ultimate lamina segments slightly expanded around the sori; plants confined to Kermadec Islands | shuttleworthianum |
| Ultimate lamina segments not expanded around the sori; plants not occurring on the Kermadec Islands | 30 | |
| 30 | Ultimate lamina segments usually <1mm wide; indusia 1–4 mm long | 31 |
| Ultimate lamina segments usually >1 mm wide; indusia 2–10 mm long | 32 | |
| 31 | Primary pinnae flattened in one plane; ultimate lamina segments 0.3–0.5 mm wide; spores 31–37 µm long, 23–27 µm wide* | hookerianum |
| Primary pinnae not flattened in one plane; ultimate lamina segments 0.5–1.0 mm wide; spores 44–49 µm long, 32–36 µm wide* | richardii | |
| 32 | Plants confined to coastal areas on Chatham Islands | chathamense |
| Plants of main islands of New Zealand | appendiculatum |
* Spore measurements are the range of mean values for sampled individuals.
A genus of around 700 species, subcosmopolitan in tropical, temperate, and subpolar regions of the world (Kramer & Viane 1990); 57 indigenous species in temperate South America (Zuloaga et al. 2008), c. 32 in southern Africa (Crouch et al. 2011), 90 in China (Lin & Viane 2013), 32 in Australia (Brownsey 1998) and c. 60 in the Pacific; 23 species in New Zealand; 10 endemic, 11 indigenous, two naturalised or casual, and one casual hybrid.
| Category | Number |
|---|---|
| Indigenous (Endemic) | 10 |
| Indigenous (Non-endemic) | 11 |
| Exotic: Fully Naturalised | 2 |
| Exotic: Casual | 1 |
| Total | 24 |
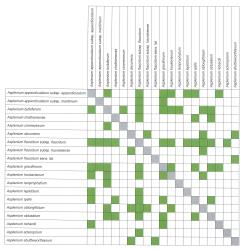
Hybridism is widespread in Asplenium, with 37 different combinations listed here, based on specimens that can be identified with reasonable certainty (Table 1). In addition there is one casual hybrid, A. ×lucrosum, (Perrie et al. 2005). Plants of hybrid origin can be identified by their abnormally formed spores. Determining parentage is much more difficult and depends not only on morphology but also on field observations of what species were growing in the immediate vicinity of the putative hybrid. Even then it is virtually impossible to determine with certainty the parentage of any particular specimen.
Brownsey (1977a) listed 19 different combinations. Additional combinations have been identified subsequently as new taxa have been described (Brownsey 1983, 1985; Brownsey & de Lange 1997; Perrie & Brownsey 2004; Perrie & Brownsey 2016) and as detailed lists of taxa have been compiled for specific areas, especially offshore islands (de Lange et al. 2011; de Lange 2015). A further eight combinations are listed here for the first time. Representative voucher specimens in AK, CHR and WELT are documented for each combination.
The taxon that hybridises most frequently is A. flaccidum subsp. flaccidum, with 11 combinations, followed by A. bulbiferum and A. gracillimum with eight combinations each. By contrast, there are five indigenous species (A. flabellifolium, A. pauperequitum, A. polyodon, A. subglandulosum and A. trichomanes) that are not known to hybridise with any other species in New Zealand and that appear to be phylogenetically distinct from those that do (see Taxonomy under Asplenium).
| Hybrid combination | Recorded by | Representative specimens | |
|---|---|---|---|
| Asplenium appendiculatum subsp. appendiculatum | |||
| ×bulbiferum | not previously recorded | AK 210852-3 WELT P011407 |
| ×flaccidum subsp. flaccidum | Brownsey 1977b | AK 159295 CHR 250240 WELT P016224 |
| ×gracillimum | Brownsey 1983 | AK 180698 CHR 446188 WELT P021336 |
| ×hookerianum | Brownsey 1977b | AK 126904 CHR 222410 WELT P016237 |
| ×lepidotum [or possibly lepidotum × lyallii] | Perrie & Brownsey 2016 | AK 30344-5 |
| ×lyallii | Brownsey 1977b | CHR 619027 WELT P016229 |
| ×richardii | Brownsey 1977b | CHR 633317 WELT P001982-3 |
| Asplenium appendiculatum subsp. maritimum | |||
| ×flaccidum subsp. flaccidum | Brownsey 1977b | AK 232178 CHR 308956 WELT P016227 |
| ×gracillimum | not previously recorded | WELT P021334 |
| Asplenium bulbiferum | |||
| ×cimmeriorum | Brownsey & de Lange 1997 | AK 252567 CHR 418942 WELT P020244 |
| ×flaccidum subsp. flaccidum | Brownsey 1977b | AK 101042 CHR308933 WELT P005128 |
| ×gracillimum | not previously recorded | AK 172974, 357229 CHR 208670 WELT P022485, P021333 |
| ×hookerianum | Brownsey 1977b | WELT P016232 |
| ×lamprophyllum | Brownsey 1977b | AK 181450 CHR 290561 WELT P016236 |
| ×oblongifolium | Brownsey 1977b | AK 134804 CHR 478432 WELT P016235 |
| ×obtusatum | Brownsey 1977b | AK 211007, AK 211008 CHR 368721 WELT P004984 |
| Asplenium chathamense | |||
| ×flaccidum subsp. flaccidum | not previously recorded | AK 300982 WELT P022277 |
| ×lyallii | Brownsey 1985 | AK 172941 CHR 403173 WELT P012511 |
| ×obtusatum | Brownsey 1985 | AK 358174 CHR 436602 WELT P027547 |
| Asplenium decurrens | |||
| ×oblongifolium | Brownsey 1977b | AK 23036, 330845 CHR 353481 WELT P009341 |
| ×shuttleworthianum | Brownsey 1977b | AK 292899 CHR 326907 WELT P001967/A |
| Asplenium flaccidum subsp. flaccidum | |||
| ×gracillimum | de Lange et al. 2011 | AK 300999 WELT P016242 |
| ×hookerianum | Brownsey 1977b | CHR 586417 WELT P025514 |
| ×lepidotum | Perrie & Brownsey 2016 | WELT P027644 |
| ×lyallii | de Lange et al. 2011 | CHR 483251 WELT P012179 |
| [AK 300982 cited by de Lange et al. (2011) from the Chatham Islands is not this hybrid but A. chathamense × flaccidum] | ||
| ×oblongifolium | Brownsey 1977b | AK 185480 CHR 219816 WELT P016238 |
| ×obtusatum | Brownsey 1977b | CHR 362100 WELT P017879 |
| ×scleroprium | Brownsey 1977b | AK 170081 CHR 359220 WELT P016230 |
| Asplenium flaccidum subsp. haurakiense | |||
| ×decurrens | not previously recorded | AK 161183-4 AK 242181 WELT P011810 |
| ×oblongifolium | Brownsey 1977b | AK 171136 CHR 308963 WELT P012487 |
| Asplenium flaccidum | |||
| ×shuttleworthianum | not previously recorded | AK 242168 CHR 418609 |
| Asplenium gracillimum | |||
| ×hookerianum | Brownsey 1977 | AK 209298 CHR 226519 WELT P016231 |
| ×lyallii | de Lange et al. 2011 Brownsey 1977b | AK 296136 CHR 195055 WELT P025550 |
| ×obtusatum | not previously recorded | WELT P021295 |
| ×richardii | not previously recorded | WELT P021335 |
| Asplenium hookerianum | |||
| ×oblongifolium | Perrie & Brownsey 2004 | WELT P020550 |
| Asplenium lyallii | |||
| ×obtusatum | Brownsey 1977b | AK 115021 CHR 308949 WELT P025243 |
Named hybrids:
Asplenium appendiculatum subsp. appendiculatum × richardii
= Asplenium ×canterburiense J.B.Armstr., Trans. & Proc. New Zealand Inst. 14: 361 (1882)
≡ Asplenium bulbiferum var. canterburiense (J.B.Armstr.) C.Chr., Index Fil. 104 (1905)
Holotype: Mount Arrowsmith, 4000 ft, J.F. Armstrong, March 1869, CHR 633317!
Brownsey (1977b) examined the holotype of Asplenium canterburiense and concluded that it was probably a hybrid between A. terrestre (= A. appendiculatum) and A. richardii, based on its morphology and aborted spores.
Asplenium bulbiferum × obtusatum
= Asplenium bulbiferum var. integra Kirk, Trans. & Proc. New Zealand Inst. 17: 232 (1885)
Lectotype (selected by Brownsey & Perrie 2017): Ulva [Island, Stewart Island], 14.12.1883, Herb. T. Kirk, WELT P004984 (on two sheets)!
Kirk (1885) described Asplenium bulbiferum var. integra from Ulva Island, Stewart Island. Syntype material from Kirk’s visit to Ulva in 1883 is in AK and WELT. All four sheets have fronds with aborted spores – probably derived from A. bulbiferum and A. obtusatum, but possibly involving A. gracillimum or A. scleroprium.
Cultivars: A number of cultivars of Asplenium are sold in the horticultural trade. Asplenium ×lucrosum is widely cultivated and usually sold as "Asplenium bulbiferum". Another common cultivar, "Maori Princess", may be a hybrid between A. bulbiferum and A. oblongifolium or A. obtusatum; it is possible that both combinations are present within "Maori Princess" as it is variable.
Incertae sedis
Asplenium bulbiferum var. decomposita Kirk, Trans. & Proc. New Zealand Inst. 17: 232 (1885)
Type: Ulva Island, T. Kirk [not located in AK, CHR, K, MEL, WELT]
Asplenium bulbiferum var. pseudolucidum Kirk, Trans. & Proc. New Zealand Inst. 17: 232 (1885)
Type: Ulva Island, T. Kirk [not located in AK, CHR, K, MEL, WELT]
Asplenium obtusatum var. pseudofalcatum Kirk, Trans. & Proc. New Zealand Inst. 17: 231 (1885)
Type: Mason Bay, Stewart Island, T. Kirk [not located in AK, CHR, K, MEL, WELT]
Asplenium tremulum Hombr. in Hombron & Jacquinot, Voy. Pôle Sud, Bot., Atlas t. 3b, f. Δ (1843)
≡ Asplenium bulbiferum var. tremulum (Hombr.) Domin, Biblioth. Bot. 20(85): 104 (1913)
Type: said to be from "Isles Mascaraignes, Tavaï, N’elle Zél’de" [not located in P]
The base chromosome number in Asplenium is x = 36 (Kramer & Viane 1990; Smith et al. 2006).