- ≡ Sphagnum javense Brid., Bot. Zeitung (Regensburg) 1: 200 (1802)
- = Dicranum candidum Brid. ex P.Beauv., Prodr. Aethéogam. 53 (1805)
- ≡ Leucobryum candidum (Brid. ex P.Beauv.) Hook.f. & Wilson in Wilson, Bot. Antarct. Voy. II (Fl. Nov.-Zel.) Part II, 64 (1854)
- = Leucobryum pentastichum Dozy & Molk., Pl. Jungh. [Miquel], 319 (1854)
- ≡ Leucobryum candidum var. pentastichum (Dozy & Molk.) Dixon, Bull. New Zealand Inst. 3: 97 (1923)
- = Leucobryum speirostichum Müll.Hal. in Geheeb, Rev. Bryol. 3: 3 (1876) nom. inval., nom. nud.
- = Leucobryum laticaule Müll.Hal., Hedwigia 36: 331 (1897)
- ≡ Leucobryum candidum var. majus A.Jaeger ex Dixon, Bull. New Zealand Inst. 3: 97 (1923) nom. nov. pro Leucobryum laticaule Müll.Hal. 1897
- = Leucobryum spinidorsum Müll.Hal., Hedwigia 36: 331 (1897)
- = Leucobryum interruptum Müll.Hal., Gen. Musc. Frond. 81 (1901) nom. nud.
Leucobryum brachyphyllum sensu Beckett (1897)
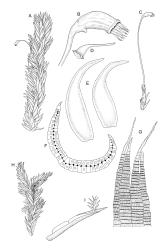
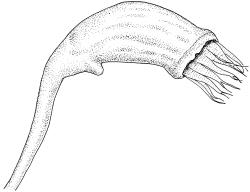
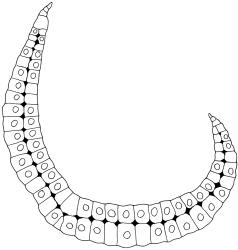
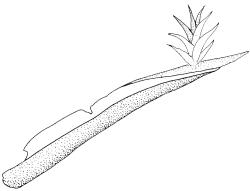
Plants extremely variable in stature and habit, white-green, pale brown-green to nearly white above and often pale brown-green below when fresh, very pale and iridescent when dry, forming small to very large dense cushions or occurring as individual stems among other bryophytes, mostly terrestrial. Stems from c.10–80 mm, often branched by forking, brown, ± brittle, in cross-section lacking a central strand, with 2–3 layers of thick-walled cortical cells; rhizoids very sparse. Leaves crowded, oblong-ovate and ± abruptly tapered to an acute apex to narrowly oblong-lanceolate, secund or erect-spreading, often in 5 strongly or weakly defined spiral ranks, strongly concave below, subtubulose above, entire, often appearing transversely corrugate, scabrous (cristate) abaxially at apex (sometimes to ⅓ of leaf) due to projecting hyalocysts, (2.8–)4.5–7.0(–11) × 1.0–1.4(–1.8) mm (under cover slip), often with smooth rhizoids arising at apices; lamina narrow and inconspicuous, in the leaf base mostly 5 or fewer cells wide. Costa nearly filling the leaf, at mid leaf consisting of a single layer of subquadrate chlorocysts surrounded by layers of hyalocysts; cross-section at widest part of the leaf base and in the upper median region with hyalocysts in one abaxial and one adaxial layer and with the abaxial hyalocysts smaller; cross-section in the alar region with hyalocysts mostly in 2–4 abaxial layers and 2–3(–4) adaxial layers, but with only one abaxial and one adaxial hyalocyst layer in the middle of the cross-section (the "isthmus" or “median furrow”), with one conspicuous round to elliptic pore (mostly 10–14 µm in greater diam.) per hyalocyst; in surface view the chlorocysts at the widest part of the leaf base rectangular and 1–2:1; alar cells neither differentiated nor auriculate.
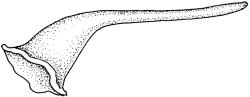
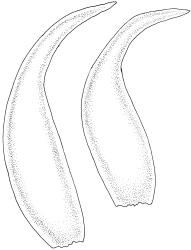
Pseudautoicous. Perichaetia lateral or on short lateral branches, aggregated in clusters, enclosing a single archegonium, the outer leaves very small, the inner leaves c. ½ the length of vegetative leaves and strongly sheathing. Dwarf males c. 2–3 mm tall, epiphytic on leaves of female plants or embedded in tomentum, with erect-spreading bracts, often enclosing only 1–2 antheridia. Setae single, variable in length, 8–22 mm, straight or slightly flexuose, weakly twisted to the left, red-brown; capsules inclined to cernuous, asymmetric, obovoid-cylindric, sulcate, strongly strumose, with a short but distinct neck, c. 1.5 mm; exothecial cells elongate and firm-walled; stomata none; annulus present, of a single row of thin-walled and persistent cells; operculum obliquely rostrate, ± equal to the capsule. Peristome teeth red, lanceolate, inserted at mouth, unequally bifid c. halfway to base, with a distinct divisural line on the outer surface, lamellate on inner surface, strongly vertically striolate below, spinose-baculate above. Calyptra c. 3 mm. Spores c. 12–15 µm, nearly smooth.
Sainsbury 1955, pl. 16 (as L. candidum); Scott & Stone 1976, pl. 26 (as L. candidum); Enroth 1990, figs. 4–7; Beever et al. 1992, fig. 22 (as L. candidum); Malcolm & Malcolm 2003, p. 41 (as L. candidum); Meagher & Fuhrer 2003, p. 27 (as L.candidum).
The nearly white plants of L. javense are unlikely to be confused with any other moss in the N.Z. flora. When growing in unusual habitats, Leucobryum javense could possibly be confused with forms of Sphagnum, but the organisation of the photosynthetic (chlorocyst) cells in a single layer within the costa will readily distinguish the Leucobryum. The pores in the hyalocyst walls are inter-cellular rather than exposed to the surface of the leaf. Confusion could also possibly occur in the field with some of the paler species of Dicranoloma (such as D. dicarpum), but the costa that nearly fills the leaf width and the clear differentiation of hyalocysts and chlorocysts in Leucobryum easily distinguish it.
K; NI: N Auckland including offshore islands (HC, LB, GB, RT), S Auckland, Gisborne, Hawke’s Bay, Taranaki, Wellington (including KA); SI: Nelson, Marlborough, Canterbury, Westland, Otago, Southland; St; Ch.
Palaeotropical? Tasmania*, mainland Australia*. Also widespread in Asia, Malesia, and western Polynesia (fide Enroth 1990, p. 78).
Terrestrial, forming large, dense cushions (to at least 0.5 m diameter). Usually best developed on tree bases and over exposed roots. Occurring in a wide range of forest types, but best developed in drier southern beech forest. Also occurring on humic banks, rotten logs and stumps, and sometimes on rock faces. Sometimes epiphytic (especially on Cyathea spp., but also on species of southern beech, Dacrydium cupressinum, Podocarpus sp., Dicksonia squarrosa, and Populus sp.). Leucobryum javense has a very broad ecological amplitude and it can occur in exotic plantations. Although typically forming hemispherical cushions, it can occur as individual strands among other bryophytes. On the North I. ranging from near sea level to c.1200 m (Mt Hauhungatahi, Wellington L.D.) and on the South I. from near sea level to at least 1120 m (Paparoa Range, Nelson L.D.).
Frequently associated species include Campylopus introflexus, Dicranoloma spp., Eurhynchium asperipes, Pyrrhobryum bifarium, Rhaphidorrhynchium amoenum, and Wijkia extenuata, and the hepatics Bazzania adnexa, B. involuta, Heteroscyphus coalitus, and Schistochila spp. (especially S. nobilis). Smaller species of Lepidoziaceae often trail among the lower leaves of Leucobryum. When it occurs over rock it is often associated with Lepyrodon lagurus. Compact and very dense cushions frequently have strongly ‘corrugate’ leaves and are associated with drier habitats. Plants laxer and more elongate in growth are usually associated with more mesic or moist microhabitats, and extreme forms are sometimes pendent from ledges or exposed tree roots.
The genus Leucobryum in N.Z. has long been a source of great taxonomic and nomenclatural confusion. Since Wilson’s (1854) moss treatment in Flora Novae-Zelandiae, the name most commonly applied to N.Z. material has been L. candidum (Brid. ex P.Beauv.) Hook.f. & Wilson. The basionym of this name, Dicranum candidum Brid. ex P.Beauv., was first validly published by Palisot de Beauvois in 1805 [Prodrome des cinquième et sixième familles de l’Aethéogamie, p. 53]. Downing & Marner (1998) discussed the type of Dicranum candidum at length and concluded that it was most likely collected by W. Dampier in 1699 in either Timor or New Guinea, rather than in Australia as stated by both Palisot de Beauvois and Enroth (1990).
In N.Z. the name L. candidum var. majus A.Jaeger has been often applied to larger and laxer plants, and L. candidum var. pentastichum (Dozy & Molk.) Dixon (or its basionym) has been applied to plants with leaves inserted in five more-or-less distinct ranks. The name L. aduncum, a species described from Java, has sometimes been applied to particularly compact and corrugate-leaved N.Z. plants, particularly those in which the three-layered “isthmus” at the leaf base is broader than usual.
The most helpful discussion of variation in N.Z. Leucobryum was provided by Dixon (1923), with Sainsbury (1955) essentially adopting Dixon’s taxonomy and nomenclature.
After studying a range of N.Z. material Dixon (1923, p. 95) stated that he had “very little hesitation … in reducing them [the Leucobryum taxa recognised from N.Z. by previous authors] all to the single type, L. candidum (Brid.) Hampe, with perhaps two fairly well-marked varieties. In all the fertile plants I have seen the fruit present no variation whatever, the length of the seta alone showing some variability, and this not correlated with any other characters, not even with the general degree of robustness of the plants. The vegetative characters are far more variable, but the internal leaf-structure is remarkably uniform throughout …” Although Dixon (1923, p. 95–98) is correct that some N.Z. forms are striking when viewed in isolation, I believe that such extreme forms are primarily environmental in nature, and that their formal taxonomic naming (at either the varietal or species level) is unwarranted.
Dixon (1923, p. 96) also presented a discussion of the leaf structure in N.Z. material. He stated that "while the leaf section in the middle and upper parts of the leaf shows a single ventral and a single dorsal layer of hyaline cells (hyalocysts), with the chlorocysts median, the basal section shows several layers (2–3) of hyalocysts on both the ventral and dorsal sides of the chlorocysts, corresponding to a considerable thickening of the leaf. This thickening, however, does not extend across the whole width of the nerve, as along the median line the hyalocysts are in two layers only, one ventral and one dorsal layer, so as to form a neck, so to speak, or isthmus, which may be of varying length". He noted that when the “isthmus” is broad, the structure approaches that of what has been termed L. aduncum Dozy & Molk. in Asia and Malesia.
With practice the multiple layers of hyalocysts at the leaf base can be visualised in surface view under the microscope or by quick sectioning under the stereoscope. The staining of leaves with toluidine blue or a similar dye may facilitate the observation of cellular structures, but in general staining is not necessary.
In a revision for the Huon Peninsula, Papua New Guinea, Enroth (1990) proposed that L. candidum (Brid. ex P.Beauv.) Hook.f. & Wilson be synonymised with the Javanese L. javense (Brid.) Mitt. He examined type material of L. javense (leg. Commerson) and discussed at length the difficulty in locating type material of Dicranum candidum. He nevertheless placed L. candidum in synonymy, arguing that he had examined “rather an extensive number of specimens (in H-BR, H-SOL, and H) which are assigned to L. candidum, and to the names given as its synonyms … from New Zealand, Tasmania, etc. Accordingly, I feel myself thoroughly familiar with these ‘species’ and their inclusion in the protean and wide-ranging L. javense seems to me inevitable.”
After the examination of a wide range of N.Z. and Australian L. candidum and a far lesser quantity of Malesian L. javense, I endorse Enroth’s (1990) concept of a broadly distributed and morphologically plastic L. javense. He stated that "even among the many variable species in this genus, L. javense is truly a bryologist’s nightmare. The only characters that remain constant in all specimens of L. javense from separate areas of its wide range are the cellular structure of the leaves and their abaxially scabrous tips. The orientation, shape, and size of the leaves and their apices exhibit exceeding plasticity." Only a single polymorphic species is accepted here for N.Z. The placement of L. candidum in the synonymy of L. javense was not accepted by Klazenga (2012).
The earliest N.Z. collection of L. javense seen is in the Turner herbarium at BM. Although labelled “New Zealand. W. [sic] Menzies. 1806”, it was probably collected in 1791 at Dusky Sound. It is representative of the species and fruiting.
Strongly developed leaf corrugations are usually associated with plants with short stems, compact colonies, and short (c. 2 mm), ± elliptic leaves. Such plants appear to be associated with particularly dry habitats. The corrugations are due to the abaxial hyalocysts being arranged in overlapping transverse rows, and they are very conspicuous in some populations. Sainsbury (1955) described such leaves as rugose.