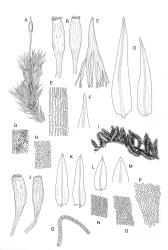
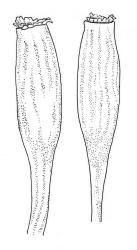
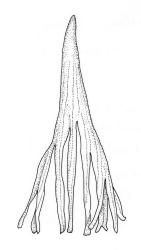
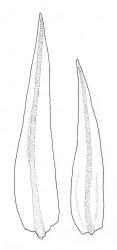
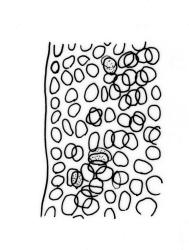
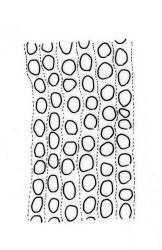
Branches variable in length, mostly 3–12 mm but sometimes to c. 35 mm. Branch leaves erect-spreading and straight or weakly flexuose when moist, strongly spiralled around the branches and with upper portions strongly reflexed when dry, narrowly to ovate-lanceolate, slenderly acuminate to narrowly long-cuspidate, keeled throughout; (1.6–)2.3–4.0 mm; upper laminal cells rounded- quadrate to oblong-elliptic (a few sometimes oblate), with conspicuous bistratose patches; inner basal cells, ± elongate-rectangular, very thick-walled and irregularly thickened; mostly c. 20–40 × 8–10 μm (occasionally shorter or longer), usually shorter and more irregular near costa. Costa mostly ending at or near the leaf apex, rarely excurrent to form a narrow cusp, often nearly filling the acumen. Setae 2.5–8(–11) mm; capsules (1.0–)1.5–2.9(–3.5) mm. Spores isosporous, 25–38 μm.
Vitt 1983, figs 1–15, 47–50 (as M. longirostre); Seppelt 2004, fig. 81 (as M. longirostre).
Of all the N.Z. Macromitria, M. longirostre var. longirostre has the upper laminal cells most conspicuously bistratose. Only M. gracile, M. longipes, and M. longirostre var. ramsayae also have areas of bistratose laminal cells, but in all cases the patches are smaller and less conspicuous.
NI: Wellington (near Cape Terāwhiti); SI: Nelson (Kōhaihai Bluff, near Cape Foulwind, Punakaikī), Westland (Jackson Bay), Otago (coastal areas), Southland (coastal areas); St; Ch; Sol; Sn; A; Ant; C; M.
Austral. Recorded by Vitt & Ramsay (1985) from Tasmania and King I. and Wilsons Promontory (Victoria) and southern South America.
On the South I. occurring exclusively in the salt spray zone both as an epiphyte and on calcareous and non-calcareous coastal rocks. The variety is not uncommon on Stewart I., and it occurs north to Jackson Bay and Dunedin. Further north it is known only from Cape Foulwind to Punakaikī (where it is common), at Kōhaihai Bluff, and near Wellington. It is very common on the southern offshore islands. Occurring from sea level to c. 30 m on the South I. but extending to higher elevations on southern offshore islands (to c. 500 m on Stewart and Auckland Is). Collections by W. Martin from higher elevations on Stewart I. are from Pryse Peak and the summit of the Tin Range, and were probably subject to salt spray despite the relatively high elevation.
When epiphytic, M. longirostre var. longirostre occurs on species of Coprosma and Pittosporum as well as on Corynocarpus laevigatus, Dracophyllum longifolium, Griselinia littoralis, Veronica elliptica, Metrosideros umbellata, Myrsine chathamica, Olearia oporina, Ozothamnus leptophyllus, Brachyglottis rotundifolia, and Weinmannia racemosa. It is also well-developed on both coastal limestone and granite. Epilithic collections at CHR are more numerous than epiphytic ones. Frequent associates (when epiphytic) include Calyptopogon mnioides, Macrocoma tenue, Macromitrium retusum, M. gracile, and Schlotheimia campbelliana. When epilithic, the variety usually forms pure or nearly pure mats.
Definitive features of M. longirostre var. longirostre include branch leaves that are spirally twisted and reflexed at their apices when dry, slightly bulging and smooth leaf cells, rather narrowly lanceolate leaves with conspicuous bistratose upper portions, and a costa ending in a keeled, acuminate-cuspidate apex. Collections from exposed rock surfaces usually have most of their upper ⅓ bistratose, while epiphytic collections often have only 2–5 rows of cells nearest the costa bistratose. Epilithic populations are also generally more compact and have shorter branches than those that are epiphytic. The stout dextrorse setae, elongate, thick-walled exothecial cells, isosporous spores, and lacerate, naked calyptrae also facilitate its recognition. Capsule shape and length are quite variable. I agree with Vitt (1983, p. 13) that male plants seem uncommon given the frequent production of capsules. I have seen males only in T. Kirk s.n. ex Campbell I. (CHR 629486). I consider perigonia to be inconspicuous, while Vitt (1983, p. 10) described them as large and bulbiform.