- ≡ Bryum capillare Hedw., Sp. Musc. Frond. 182 (1801)
- = Bryum searlii R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 459 (1899)
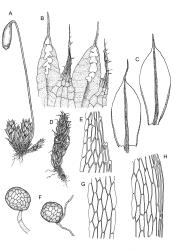
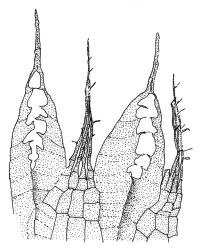
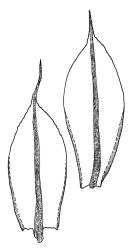
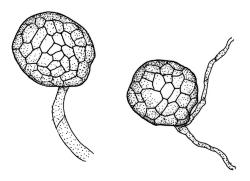
Plants medium-sized, bright green or rarely pink, lustrous, moderately comose, forming loose turves. Stems red-brown, c. 7–12 mm, branching by subperichaetial innovation in fertile material (sterile plants unbranched), beset below with pale red-brown, moderately papillose rhizoids, in cross-section as per genus. Leaves evenly spaced on sterile stems, ± comose and with lower stems often ± leafless in sexual plants, erect-spreading when moist, weakly to strongly twisted around the stem when dry, obovate-spathulate, widest above the middle, and ± narrowed at base, rather abruptly tapered to an elongate awn (less often a cusp), not reflexed apically, to 3.5 × 1.5 mm and with lamina 0.8–0.9 the total leaf length, bordered throughout, recurved on both sides, weakly concave, denticulate above or entire, not decurrent; upper laminal cells hexagonal-rhombic, thin- or rarely ± firm-walled, not or weakly porose, c. 50–60 × 18 µm and mostly 2–4:1, little altered near apex, and more oblong towards leaf base; marginal cells linear and firm-walled to form a distinct border of c. 2–4 cells at widest part of leaf and extending ± to apex; basal cells mostly not pigmented. Costa concolourous with lamina or red-brown, variably excurrent, mostly to form a long, ± denticulate awn. Brood bodies (axillary gemmae and/or tubers) sometimes present; axillary gemmae rare in N.Z. material, filamentous, chocolate-brown; tubers often present in N.Z. material, globose or ± irregular, mostly 180–260(–360) µm in greater diam., pale brown, with cells not protruding.
Synoicous or polygamous. Perichaetia near plant base; perichaetial leaves smaller than vegetative, ± lanceolate and entire with a long-excurrent costa. Perigonia terminal and ± comal, the bracts not differentiated, enclosing numerous antheridia and 7–9-celled filiform paraphyses. Setae single, c. 20–40(–55) mm, ± red, cygneous just below capsule; capsules pendent, clavate-cylindric, 3–5 mm long, with an ill-defined neck that is c. ⅓ the total length and weakly wrinkled when dry; operculum low-conic, apiculate. Exostome teeth pale; endostome with very broad, fenestrate segments that taper abruptly to a filiform apex and 3–4 appendiculate cilia. Spores 11–14 µm, smooth.
Syed 1973, figs. 1–4 (as Bryum capillare var. capillare); Eddy 1996, fig. 415 (as B. capillare); Spence & Ramsay 2006, fig. 47, a–h.
Collections of R. capillare with only weakly excurrent costae are best distinguished from R. subtomentosum by the non-reflexed leaf apices, the thinner-walled and non-porose nature of the laminal cells, and the smaller tubers of the former. The dry leaves of R. capillare are spiralled around the stem, whereas those of R. subtomentosum tend to twist around their own axis when dry.
Rosulabryum capillare is best distinguished from R. campylothecium by features discussed under the latter species. The potential for confusion between R. capillare and Bryum creberrimum is discussed under the latter species.
NI: N Auckland (Waipū, Tī Point, Woodhill), S Auckland (Waitomo), Gisborne (Panikau), Hawkes Bay (Wairoa, Māhia Peninsula, Puketīriri, Pētane, Havelock North), Wellington (Mt Ruapehu, Wanganui, Ruahine Range, Carterton); SI: Nelson (Mt Owen), Marlborough (Kenepuru, Kaikōura, Wairere Stream, Haldon Hills), Canterbury (Waipara, Akaroa), Otago (Ōamaru, Taieri River, Alexandra, Remarkable Range, Tautuku Beach); St; Ch.
Nearly cosmopolitan. The world distribution is summarised by Syed (1973) to include Europe, eastern and western North America including Mexico, South America, Hawai’i, Japan, Australia, New Zealand, South Africa, central Africa, Morocco, and Canary Islands.
On soil (often humic) over a variety of substrates, including concrete, limestone, and logs. Mostly terrestrial in base-rich situations but occasionally epiphytic. Recorded from sand hills on the Māhia Peninsula. Ranging from sea level to at least 1220 m. Associated species include Bryum caespiticium, B. dichotomum, Camptochaete pulvinata, Ceratodon purpureus, Fabronia australis, and Tortella knightii.
The spathulate, weakly comose, and bordered leaves, which are strongly spiralled around the stem when dry, are usually sufficient for recognition. The thin-walled laminal cells and strongly excurrent costa provide further distinction.
Syed (1973) considered the segregate Bryum torquescens Bruch to occur in N.Z. He differentiated B. torquescens from R. capillare s.s. on the basis of synoicous sexuality and orange to red tubers in the former. Spence & Ramsay (2006) recorded this species (as a Rosulabryum) as widespread in Australia, but did not record it from N.Z. There is no clear boundary in N.Z. between dioicous and synoicous material. Syed's (and Spence & Ramsay’s) narrowly defined segregates from R. capillare cannot easily be applied here. The great majority of N.Z. collections are synoicous (i.e., with some antheridia in seta-bearing perichaetia) but antheridial plants can also be found in some collections. Although some collections appear dioicous, perichaetia can usually be found with at least a few antheridia present. A collection from Rangitoto I., North Auckland L.D. (L.H. Millener 39, WELT M012001) provides a good example of polygamous, fruiting material. Tubers are observable in most, but not all, collections; none have been seen that are orange or red. The most tenable taxonomic position is to apply the name R. capillare to all N.Z. material, while noting its variable sexuality.
The name Bryum obconicum Hornsch. is applied to numerous collections in N.Z. herbaria; these specimens are referred here to R. capillare. The former name is discussed very briefly above in the "Excluded Taxa" of Bryum.
Distinctive material allied to R. capillare occurs on the Port Hills of Canterbury L.D. This compact plant is apparently dioicous, with linear-lanceolate and entire inner perichaetial leaves and has firm-textured, short (c. 2 mm) elliptic to obovate, acute and non-decurrent leaves with stout, percurrent costae, and very weakly bordered, denticulate margins. Rhizoids of this population bear numerous, ± globose, red-brown tubers and the leaf axils bear abundant chocolate brown, filamentous, unbranched, and coarsely baculate-papillose gemmae. The presence of axillary filamentous gemmae is used by Syed (1973) to partially define several segregate species allied to R. capillare, but the Port Hills population does not agree with any of his segregates.