- ≡ Trichostomum perichaetiale Hook., Musci Exot. 1, pl. 73 (1818)
- ≡ Sprucea perichaetialis (Hook.) Hook.f. & Wilson, Bot. Antarct. Voy. I. (Fl. Antarct.) Part I, 128 (1845)
- ≡ Symblepharis perichaetialis (Brid.) Hook.f. & Wilson in Wilson, Bot. Antarct. Voy. II (Fl. Nov.-Zel.) Part II, 60 (1854)
- = Trichostomum moretonii R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 483 (1897)
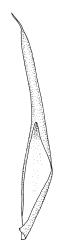
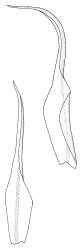
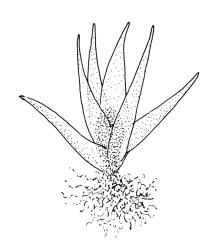
Plants medium-sized, bright yellow-green, forming cushions. Stems branched by sparse forking and by subperichaetial innovation, c. 8–25(–35) mm, often with microphyllous shoots in axils of upper leaves, in cross-section with a small central strand, densely tomentose with pale brown, smooth, strongly contorted and branched rhizoids. Leaves moderately twisted and ± inrolled from an erect and weakly sheathing base when moist, strongly contorted and inrolled (occasionally cork-screwed) when dry, slightly but distinctively lustrous when dry, rather abruptly tapered from an obovate-elliptic base to an acuminate apex, entire throughout or crenulate to very weakly toothed at shoulder, c. 3.0–4.5 × c. 0.9 mm, weakly sheathing below, U-shaped in cross-section above, plane at margins, with lamina at mid leaf distinct; mid laminal cells short and rounded, distinct in surface view, mostly 5–12 × 5–8 µm and 1–1.5:1 (but often some cells oblate, especially near margins), incrassate, smooth, mostly unistratose but bistratose at upper margins and often completely bistratose near apex; in cross-section with cell walls bulging on both surfaces, transitioning gradually to the cells of the leaf base; cells of the leaf base linear or narrowly oblong, incrassate and porose; alar cells yellow-brown, forming a large, well-defined group extending c. 6–8 cells up the margin and ± to the costa, oblong, inflated but firm-walled. Costa filling c. ⅙ or less of the leaf base, percurrent or nearly so, not filling the upper leaf, in mid leaf cross-section rounded on abaxial surface, with c. 5 median guide cells and large abaxial and adaxial stereid groups, with cells of the exposed adaxial layer (at mid leaf & below) similar to contiguous stereids and lacking a distinct lumen; in adaxial surface view (at mid leaf) cells elongate.
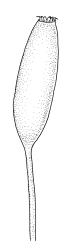
Dioicous (usually pseudautoicous). Perichaetial leaves sheathing the setae, each with a long, strongly sheathing oblong base and abruptly tapered to a narrow subula c. ½ or less the total leaf length, weakly costate, c. 5.5–7(–9) mm. Dwarf males frequent in leaf axils of ♀ plants, c. 0.5–1 mm, often with multiple perigonia (♂ plants rarely normally developed). Setae c. 15–30(–40) mm, ± straight, pale brown or yellow; capsules erect or slightly curved, oblong-cylindric, slightly narrowed to mouth, 2.0–2.6 mm, smooth, straw-coloured; exothecial cells irregular in outline, not in distinct columns, with moderately and uniformly thickened walls; stomata few at extreme base of capsule, superficial; operculum long rostrate, equal to or somewhat longer than the capsule. Peristome teeth inserted c. 75 µm below the rim, paired, undivided or weakly divided to about ⅓ of their length, c. 150–180 × c. 40 µm, mostly blunt at apices, strongly papillose-baculate throughout (apparently on both surfaces). Calyptra c. 5 mm, clasping at base. Spores 14–21 µm, spherical, thick-walled, finely papillose.
Hooker 1818, pl. 73 (as Trichostomum perichaetiale); Ramsay 1986, figs. 1–22; Meagher & Fuhrer 2003, p. 157; Malcolm & Malcolm 2003, p. 34; Klazenga 2006, fig. 2 c–d.
The contorted leaves of H. perichaetiale give it a very different aspect, both when moist and when dry, than H. trichopodum, and these two species are unlikely to be confused; they may be distinguished by many features cited in the key above.
Holomitrium perichaetiale could be confused with Dicranoweisia antarctica, particularly when the Holomitrium occurs on rock. The Holomitrium has broader, more distinctly shouldered leaves, which are generally inrolled towards the stem when dry, while in Dicranoweisia antarctica the narrower leaves lack a distinct shoulder and are twisted in a corkscrew manner when dry. Holomitrium perichaetiale normally has epiphytic dwarf ♂ plants, while D. antarctica is autoicous. Both species have perichaetial leaves sheathing the setae, but those of H. perichaetiale are longer (mostly 5.5–7 mm) and very abruptly tapered to a setaceous apex, while those of D. antarctica are c. 2.5 mm and gradually tapered. The absence of cuticular striations in H. perichaetiale can be useful in difficult instances.
Confusion sometimes occurs between H. perichaetiale and species of Tortella, but all species of the latter genus have very shiny costae when dry. These contrast sharply with the matt green lustre of both the costae and the laminae in H. perichaetiale. Also, the gradual transition between the cells of the leaf base and the mid laminal cells of H. perichaetiale are distinct from the usually abrupt and V‑shaped boundary between the basal and mid laminal cells in Tortella. In the present species the mid to upper laminal cells are distinct and smooth in surface view, while in species of Tortella mid to upper laminal cells are obscure and pluripapillose. The normally epiphytic substrate of H. perichaetiale contrasts with the usually terrestrial or rock substrate of species of Tortella.
NI: N Auckland, including offshore islands (PK, HC, LB, GB), S Auckland, Gisborne, Taranaki (Mt Messenger, Mt Egmont), Wellington; SI: Nelson, Marlborough (Kenepuru Sound, Pelorus Bridge Scenic Reserve), Canterbury, Westland, Otago, Southland; St; Ch; A; C.
Australasian. Tasmania*, eastern mainland Australia*, New Caledonia*.
On trunks and branches of a wide range of coniferous and dicotyledonous trees, including the genera Agathis, Dacrydium, Dacrycarpus, Halocarpus, and Podocarpus, as well as Coprosma, Dracophyllum, Fuchsia, Fuscospora, Hoheria, Leptospermum, Lophozonia, Pseudowintera, Sophora, and Weinmannia. Also commonly occurring on rock, rotting logs, and occasionally on humus. Epilithic populations form loose cushions or turves to at least 12 cm diam. and are found mainly at higher elevations (above c. 1000 m). A wide range of epiphytic and epilithic bryophyte species commonly grow in association with this species. From near sea level to at least 1200 m (Mt Ruapehu, Wellington L.D., and Mt Owen, Nelson L.D.) on both main islands.
The dry plants have a slight but distinctive lustre, which, combined with the strongly contorted leaves and conspicuous (but not shiny) costa, make the species easy to recognise, in the both field and the herbarium. When present, the sheathing and abruptly setaceous perichaetial leaves and ± erect cylindric capsules with short and paired peristome teeth provide further distinction.
Unsurprisingly, given its wide geographic range and substrate tolerance, H. perichaetiale shows considerable variability. In most populations the upper leaf lamina is unistratose, except for a narrow bistratose border. However, the uppermost 300–400 µm is often entirely bistratose, and areas of bistratose lamina can be observed at mid leaf in many populations. Rarely, specimens are seen in which the upper half of the lamina is predominantly bistratose (e.g., W. Martin 260.20 from Carterton, CHR 532209). Some populations (particularly on Stewart I. and the Auckland Is) have leaves less distinctly shouldered than usual for the species. Plant stature also varies considerably. Ramsay (1986) published observations on variability in H. perichaetiale based on N.S.W. material, paying particular attention to leaf and stem dimensions.
The microphyllous shoots can be very abundant, often occurring in clusters of 10–12 in the axils of adjacent leaves. Individual microphyllous shoots are mostly 2–4 mm long and bear acute leaves c. 1–2 mm long. Microphyllous shoots are most common and best developed in populations from above 1000 m.
Dwarf, epiphytic ♂ plants are readily seen in fruiting collections of H. perichaetiale. In one fruiting N.Z. specimen (B.H. Macmillan 77/25 from Donald Creek, Nelson L.D., CHR 267524), ♂ plants equal in size to ♀ plants have been observed. In other respects this material is representative of the species. A single specimen from New Caledonia (M.R. Crosby 14025, NY) with ♂ plants equal in size to ♀ plants has also been seen. In a cytological study of H. perichaetiale, Ramsay (1986) investigated the factors affecting the production of dwarf ♂ plants. She reported a chromosome number of n = 10 and the presence of a large and "slightly dimorphic" bivalent, which disjoined early in meiosis, similar to that occurring in some pseudautoicous species of Dicranum and Dicranoloma. She found only dwarf males in H. perichaetiale.
Dixon (1923, p. 78) tentatively suggested that the Queensland H. muelleri Hampe [Linnaea 36: 514 (1870)] should be considered synonymous with H. perichaetiale. Dixon’s suggested synonymy was accepted by Wijk et al. (1962) and by Streimann & Klazenga (2002). Type material from Rockingham Bay collected by F. Mueller (NY-Jaeger!) has mid leaf margins 3–4 cells thick and distinctly winged in cross-section. A second specimen from Ballina, N.S.W. (W.W. Watts, CHR 642129) has similar leaf margins. These observations suggest that H. muelleri deserves recognition.
Holomitrium perichaetiale has been recorded from Lord Howe I. (Brotherus & Watts 1915; Streimann & Klazenga 2002), but material from there is different in a number of ways from N.Z. material and it might profitably be compared to Polynesian H. vaginatum (Hook.) Brid. Material of H. perichaetiale var. robustum Broth. & Watts [Proc. Linn. Soc. New South Wales 40: 365 (1915)] has not been studied.