- ≡ Hypnum laxatum Mitt., Hooker's J. Bot. Kew Gard. Misc. 8: 264 (1856)
- = Hypnum elusum Mitt. in Hooker, Handb. New Zealand Fl. 478 (1867)
- = Rhynchostegium peracuminatum Dixon & Sainsbury in Sainsbury, Trans. & Proc. Roy. Soc. New Zealand 75: 185 (1945)
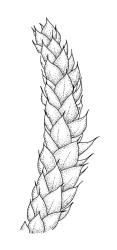
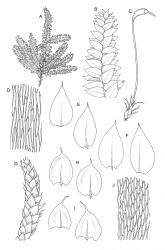
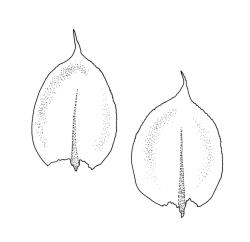
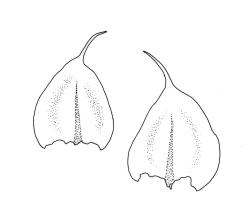
Plants small to medium-sized, yellow‑green or occasionally bright green, weakly lustrous. Stems irregularly to subpinnately branched, c. 20–50 mm long, decumbent, in cross‑section with 3–5 layers of thick-walled outer cells and a distinct central strand. Branches ascendant and short (mostly 6–8 mm). Stem and branch leaves differentiated by shape, when dry ± striolate and erect, erect-spreading and not or weakly striolate when moist. Stem leaves broadly ovate to oblong-ovate, abruptly tapered to a narrowly acuminate or filiform apex (acumen mostly 0.45–0.6 mm and up to c. 0 4 the total leaf length), weakly concave, narrowed to the insertion, erect to spreading, weakly decurrent, 1.4–1.9 × 0.6–1.0 mm (including acumen), entire or denticulate above; mid laminal cells linear-rhombic, smooth, firm-walled, mostly 54–90 × 7–9 µm; cells of the acumen not differentiated; cells at insertion shorter, thicker-walled and porose in several rows; alar cells slightly inflated or subquadrate in a poorly differentiated group. Branch leaves ovate-acuminate, the acumen usually narrow and ± piliferous, mostly 1.2–1.6(–1.8) × 0.4–0.55(–0.65) mm, erect-spreading when moist, erect and striolate when dry; mid laminal cells linear-rhombic, firm-walled, mostly 45–60(–90) × 7–9 µm, becoming longer in lower leaf and shorter and broader at insertion; alar cells subquadrate and mostly weakly inflated, forming a medium-sized but poorly defined group. Costa variable in length, single or rarely slightly forked, (⅓–)½–⅔ the leaf length, terminal spine lacking or rarely rudimentary.
Perichaetia c. 2.0 mm long, the inner leaves acuminate from an oblong base and erect. Perigonia scattered on stems and branches, c. 0.6–0.8 mm, paraphyses not seen. Setae flexuose, sinistrorse, 12–15 mm, red-brown, smooth; capsules oblong-cylindric, red-brown, constricted below the mouth when dry, 1.4–1.8 mm; exothecial cells oblong, firm-walled, distinctly or weakly collenchymatous; annulus adhering to the operculum; operculum c. 1 mm, curved and distinctly shorter than the theca. Exostome teeth as genus, c. 450 µm; endostomal segments perforate, cilia single or paired, nodose. Spores 13–15 µm, green.
Many herbarium specimens of R. muriculatum are misnamed as R. laxatum. When sterile, the most useful character for distinguishing the two species is the width of the stem leaves, which are c. 0.6–1.0 mm wide in R. laxatum and mostly 0.4–0.5 wide in R. muriculatum. The smooth setae of R. laxatum will readily distinguish fertile material.
NI: N Auckland, S Auckland, Gisborne, Hawke’s Bay, Taranaki, Wellington; SI: Nelson, Marlborough, Canterbury, Westland, Otago, Southland; St; Ch; Sol; Sn; A. Reported from C by Vitt (1974).
Australasian. Tasmania*, mainland Australia*. Hedenäs (2002) has detailed the occurrence of this species in Australia.
Mostly on soil, rotten logs, and tree bases, but also occurring on rock (especially basalt and limestone) and epiphytic on a variety of trees and shrubs, including Coprosma grandifolia, Hoheria sp., Melicytus ramiflorus, Metrosideros excelsa, and Myoporum laetum. Occurring in a range of indigenous forest, shrubland, and grassland types. Also occurring in disturbed habitats, and either under, or epiphytic on, a range of exotic trees (including Eucalyptus, Pinus, Populus, Quercus, Salix, and Sambucus), including those in plantations. On the North I. ranging from near sea level (near Foxton, Wellington L.D.) to at least 900 m (near Blyth Track, Wellington L.D.), and on the South I. to c. 1230 m (Mt Mytton, Nelson L.D.). Relatively few records are from above 1000 m, and these all come from limestone or marble ledges in Nelson L.D.
Occurring with a wide range of terrestrial, epilithic and epiphytic species, including Alleniella hymenodonta, Eurhynchium asperipes, Orthorrhynchium elegans, Racopilum strumiferum, Rhaphidorrhynchium amoenum, Rhynchostegium muriculatum, R. tenuifolium, and Weymouthia cochlearifolia. Mixtures of Rhynchostegium spp. are common and often confusing.
Representative material of R. laxatum is easily recognisable. The distinctly striolate (when dry) and non-complanate branch leaves, which are abruptly and usually narrowly acuminate, give this species a distinctive facies. Given its autoicous sexuality, at least some sporophytes are usually observable; the smooth seta and relatively short operculum help to differentiate this species from its congeners. Differences in habit are as reliable as the length of laminal cells to differentiate the present species from R. tenuifolium, despite considerable variability related to habitat conditions.
In the lectotype specimen of this species the upper laminal cells (in branch leaves) are mostly 54–75 µm long; mid to lower laminal cells have similar dimensions, and the leaves are mostly c. 1.2 mm long. These measurements are all near the lower end of the continuous variation for the species.
Although well-developed and representative material is easily recognised, this is a highly variable species and not all material can be named with confidence.
After initial uncertainty, I concur with Sainsbury (1955, p. 443) that R. peracuminatum Dixon & Sainsbury is referable here. The type specimen (three isotypes seen) has branch leaves at the upper end of continuous variation for this species (to c. 1.8 × 0.5 mm) and the specimen is brighter yellow and more lustrous than usual for R. laxatum; most mid laminal cells (branch leaves) are c. 75–90 µm long and the dry leaves are striolate. Like Sainsbury, I consider these specimens to represent an extreme form of R. laxatum.