- ≡ Hypnum smithii Hedw., Sp. Musc. Frond. 264 (1801)
- = Leptodon novae-seelandiae Müll.Hal., Hedwigia 41: 131 (1902)
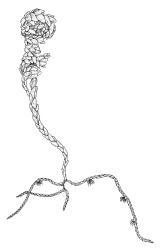
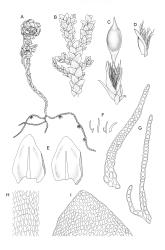
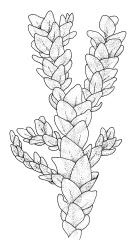
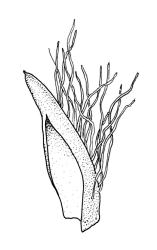
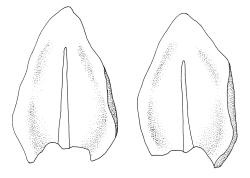
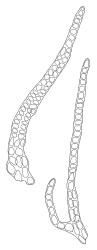
Plants dark green, with secondary stems erect, strongly inrolled when dry, and arising from a stoloniferous primary stem. Primary stems pale brown, irregularly branched, with scale-like leaves and scattered fascicles of smooth brown rhizoids, in cross-section with 3–4 layers of thick-walled cortical cells and no central strand. Secondary stems erect, once or twice irregularly to subpinnately branched, 10–25 mm (rarely to c. 40 mm in shaded forms), strongly curled upwards and inrolled when dry, curved when moist, with branches to c. 8 mm, simple or subpinnately branched, occasionally with few to many attenuate and microphyllous branches. Leaves of secondary stems and branches variable in size but otherwise similar, mostly (0.42–)0.48–0.70(–0.90) × (0.21–)0.30–0.48(–0.60) mm and c. 3:2 l:w (excluding those of attenuate branches), appressed when dry, spreading when moist, loosely complanate, broadly ovate or elliptic, mostly asymmetric, broadly rounded at apex, cordate or weakly decurrent, usually slightly auriculate on one side, concave and often with a single plica on each side of costa, with margins entire or weakly crenulate near apex and inrolled in lower half; upper laminal cells rhombic-elliptic, mostly 9–15 × 5–7 µm, firm-walled, smooth, somewhat smaller and more quadrate near margins, scarcely altered in lower leaf; alar cells forming a large but weakly defined group of small, ± opaque, and quadrate to ± oblate cells, sometimes with c. 10–20 slightly enlarged and irregular cells in extreme angles. Costa single, stout, unbranched or with a small lateral spur, extending c. ¾ the leaf length. Paraphyllia linear or strap-shaped, mostly 2–4 cells wide, c. 100–200 µm long, some with lateral teeth or appendages. Pseudoparaphyllia not seen. Brood bodies and other asexual propagules absent.
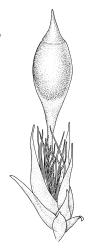
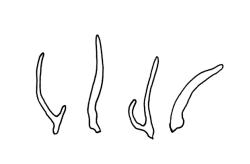
Dioicous. Perichaetia scattered on secondary stems and branches, with leaves c. 1 mm long at archegonial maturity, weakly costate, lanceolate and tubular, after fertilisation becoming greatly elongate (to c. 3 mm) and sheathing the seta base. Perigonia scattered on secondary stems, c. 0.9 mm long, with inner bracts more elongate than outer, tubulose, ecostate, enclosing 2–3 antheridia and few filiform paraphyses. Setae short (c. 3 mm including vaginula), yellow-brown; vaginula densely covered with very long (c. 2.5 mm) golden filaments that extend to or slightly beyond the capsule base; capsules erect or inclined, short-exserted, ellipsoid, c. 2 mm, yellow-brown; exothecial cells rather thin-walled, mostly oblong, several rows smaller and rounded at rim; stomata few and difficult to observe, restricted to extreme capsule base; operculum obliquely rostrate from a conic base, 0.8 mm. Exostome teeth linear-lanceolate, papillose throughout, c. 300 µm; endostome rudimentary. Calyptra cucullate, c. 2 mm, with numerous golden hairs at base and usually extending beyond apex. Spores ellipsoid or spherical, variable in size in a single capsule, mostly 12–30 µm, coarsely but lowly insulate, thick-walled.
Brotherus 1925, fig. 566; Magill & van Rooy 1998, fig 163, 12–24; Smith 2004, fig. 237, 1–3.
NI: N Auckland (Whangārei, Woodhill), including offshore islands (GB), S Auckland, Gisborne, Hawke’s Bay, Wellington; SI: Nelson, Marlborough, Canterbury, Otago, Southland; Ch (Tennant’s Lake, Pitt I.). There are no confirmed records from Taranaki L.D. and the only collections from the West Coast are from limestone outcrops in/near Punakaikī and from coastal divaricating shrubs at Karamea (both localities in Nelson L.D.). In the Mt Arthur-Cobb Valley area (Nelson L.D.) this species is restricted to marble outcrops. It is more frequent in the drier portions of both main islands.
Anomalous. Australia*, Africa*. Recorded from southern and western Europe including Britain by Smith (2004, p. 719). Recorded also from south-west Asia, several regions of Africa, southern South America, and Juan Fernández Is by Magill & van Rooy (1998) and North America by Anderson et al. (1990).
Occurring on a wide range of tree species, excluding species of southern beech (although it sometimes occurs in southern beech-dominated forests). Also on dry or mesic rock (limestone, marble, schist, or basalt). Recorded host species include Carpodetus serratus, Coprosma areolata, Corynocarpus laevigatus, Dysoxylum spectabile, Elaeocarpus hookerianus, Griselinia littoralis, Hoheria angustifolia, Leptospermum scoparium, Lophomyrtus obcordata, Melicytus ramiflorus, Nestegis cunninghamii, Pennantia corymbosa, Pittosporum eugenioides, Pseudopanax arboreus, Sophora sp., Veronica elliptica, Vitex lucens, and Weinmannia racemosa as well as the gymnosperms Dacrycarpus dacrydioides, Podocarpus totara, and Prumnopitys ferruginea. Epiphytic associates include the mosses Fabronia australis, Macromitrium retusum, Neckera laevigata, Orthorrhynchium elegans, Syntrichia papillosa, and Weymouthia cochlearifolia, the hepatics Porella elegantula and Frullania spp., and gelatinous lichens. Epilithic associates include Austrohondaella limata, Fallaciella gracilis, Lembophyllum divulsum, Palamocladium sericeum, Schistidium apocarpum s.l., Macromitrium retusum, and Syntrichia serrata, as well as Frullania spp. and gelatinous lichens. On North I. ranging from near sea level to c. 520 m elevation (Sentry Box Scenic Reserve, Hawke’s Bay L.D.) and on South I. from near sea level (Punakaikī, Nelson L.D.) to 1360 m elevation (Black Birch Range, Marlborough L.D.).
Material of L. smithii from N.Z. is generally more compact than both European and east African collections, but compares well to them in other respects.
The strongly inrolled dry branches of this species are highly distinctive. Stout but short costae, broadly ovate and apically rounded leaves, and epiphytic or epilithic habitat also facilitate the recognition of L. smithii in the field. Under the microscope, the presence of a rudimentary endostome readily distinguishes it from members of the Neckeraceae, the family where it was placed by Sainsbury (1955). Among the N.Z. representatives of the Neckeraceae, only Alleniella hymenodonta (Müll.Hal.) S.Olsson, Enroth & D.Quandt (Neckera hymenodonta Müll.Hal.) has paraphyllia.
The type of L. novae-seelandiae bears a large number of microphyllous and attenuate branches (with leaves mostly c. 0.30 mm or shorter and c. 3:2 l:w and with reduced paraphyllia), but otherwise is not distinctive. It is not deserving of taxonomic separation. The microphyllous state of this collection is perhaps related to the deposition of wind-blown sand on the colony, which Beckett (in herb.) described as "1 to 1½ inch" deep. However, other specimens, (e.g., D. Glenny s.n. from Port Levy, Canterbury L.D., CHR 438699 and W. Martin 251.15 from Waihopai River, Marlborough L.D., CHR 536755), exhibit a similar tendency to produce microphyllous branches. In addition to depositional habitats, microphyllous branches appear to be associated with strongly shaded conditions.
Populations of L. smithii producing microphyllous branches resemble the rare epiphyte Scorpiurium cucullatum. The branch leaves of well-developed L. smithii are broader in relation to their length (c. 3:2 l:w, excluding those of attenuate branches) than the secondary stem and branch leaves of S. cucullatum (c. 2:1 l:w). Even in the microphyllous branched forms of Leptodon, the leaves are more rounded apically than the broadly acute or obtuse apices of S. cucullatum and mid laminal cells are less rhombic and shorter in the Leptodon (<15 µm vs c. 24–30 µm). The strap-shaped paraphyllia of L. smithii are present even on microphyllous branches while they are lacking in Scorpiurium.